Abstract
-
Purpose
This review examines the significance of perioperative nutritional management in organ transplantation, with a particular focus on liver transplantation. Organ transplant recipients often experience malnutrition and sarcopenia due to nutritional and metabolic abnormalities associated with organ dysfunction. Because transplantation is a highly invasive procedure, optimizing perioperative nutritional care is critical for improving short-term outcomes and reducing postoperative infection-related mortality.
-
Current concept
Recent clinical investigations have shown that liver transplant recipients, who are frequently afflicted with end-stage liver disease and uncompensated cirrhosis, are particularly vulnerable to protein-energy malnutrition and secondary sarcopenia. Our analysis identified low pre-transplant nutritional status and the absence of preoperative branched-chain amino acid supplementation as independent risk factors for post-transplant sepsis. In response, we developed a customized nutritional therapy protocol that incorporates precise body composition analysis, serial measurements of biochemical markers (including prealbumin, zinc, and the branched-chain amino acid/tyrosine ratio), and targeted supplementation with branched-chain amino acids, zinc acetate, and synbiotics. Early initiation of enteral nutrition coupled with postoperative rehabilitative interventions resulted in improved outcomes. In addition, stratified body composition parameters correlated with survival differences and informed revised transplantation criteria.
-
Conclusion
Tailored perioperative nutritional management and rehabilitative strategies are essential for improving early postoperative outcomes in liver transplantation. These findings underscore the need for proactive nutritional assessment and intervention, which may represent a breakthrough in transplant prognosis. Future research should refine nutritional protocols and integrate novel biomarkers, while education and interdisciplinary collaboration remain crucial for enhancing transplant outcomes and reducing complications.
-
Keywords: Enteral nutrition; Liver transplantation; Nutrition assessment; Protein-energy malnutrition; Sarcopenia
Introduction
Background
Organ transplantation encompasses procedures involving the heart, lung, liver, pancreas, kidney, and small intestine. Consequently, organ transplant recipients frequently experience malnutrition and sarcopenia due to nutritional and metabolic abnormalities associated with organ dysfunction. Additionally, because organ transplantation is a highly invasive surgical procedure, effective perioperative nutritional management is critical for patients to tolerate the surgery. Thus, perioperative nutritional management is essential for improving short-term prognosis in organ transplant patients.
Objectives
In this article, I discuss the significance of perioperative nutritional management in organ transplantation and its role in improving prognosis, using liver transplantation—my specialty—as an illustrative example drawn from my own experience.
Ethics statement
This is a literature-based study. Institutional Review Board approval was not required, as the study did not involve human subjects research.
Necessity of perioperative nutritional management in liver transplantation
Liver transplant recipients suffer from end-stage liver diseases that cannot be managed by internal medicine or alternative surgical methods. These conditions include biliary atresia, biliary stasis, various hepatocellular diseases (e.g., viral or alcoholic cirrhosis), hepatocellular carcinoma complicated by liver cirrhosis, and acute liver failure. The progression of these diseases to decompensated cirrhosis often results in protein-energy deprivation. Moreover, many liver transplant recipients develop secondary sarcopenia. In addition, liver transplantation is considered a high-risk procedure because the immunosuppressive drugs used postoperatively increase the risk of infection.
I became involved in liver transplantation in 2007. At that time, I observed a steep decline in the post-transplant survival curve during the early postoperative period, indicating a high early post-transplant mortality rate. Consequently, I concluded that reducing early post-transplant mortality was essential to improve outcomes and to understand the causes of early post-transplant death. Our analysis revealed that more than 60% of deaths were due to infectious diseases such as pneumonia and bacteremia [
1]. We identified low pre-transplant nutritional status (as measured by low body cell volume) and the absence of preoperative branched-chain amino acid supplementation as independent risk factors for post-transplant sepsis and infection-related mortality [
2]. Furthermore, we observed that the preoperative nutritional status of liver transplant recipients ranged from relatively well-nourished patients to those with markedly reduced muscle mass and nutritional deficiency. Thus, we developed a treatment strategy emphasizing customized perioperative nutritional intervention, infection control, and improved short-term post-transplant outcomes.
Establishment of tailor-made perioperative nutritional therapy
Accurate nutritional assessment and appropriate nutritional therapy are the two cornerstones of effective nutritional management. However, common assessment parameters such as body mass index, brachial circumference, and serum albumin are inadequate for evaluating liver transplant patients with cirrhosis and edema. Therefore, we implemented body composition analysis using a specialized analyzer to accurately assess the nutritional status of liver transplant recipients. This evaluation revealed that preoperative hyponutrition, as indicated by low somatic cell volume, is an independent risk factor for early post-transplant mortality due to infection [
2].
In addition, we measured blood biochemical nutritional parameters over time, including prealbumin (transthyretin), a rapidly turning over protein, zinc, and the branched-chain amino acid/tyrosine ratio. The findings indicated that levels of prealbumin, zinc, and the branched-chain amino acid/tyrosine ratio were markedly decreased at admission [
3]. Zinc, in particular, demonstrated a significant positive correlation with prealbumin and a negative correlation with ammonia [
3]. Hypozincemia is linked to delayed wound healing, stomatitis, decreased appetite, hypoproteinemia, and hyperammonemia, all of which impede early postoperative recovery. Liver transplantation requires anastomosis of blood vessels and bile ducts, and hypozincemia occurs in the early postoperative period when wound healing is most important and appetite should be improved [
4]. Therefore, we measured serum zinc levels and considered perioperative zinc supplementation to be necessary if zinc levels were low.
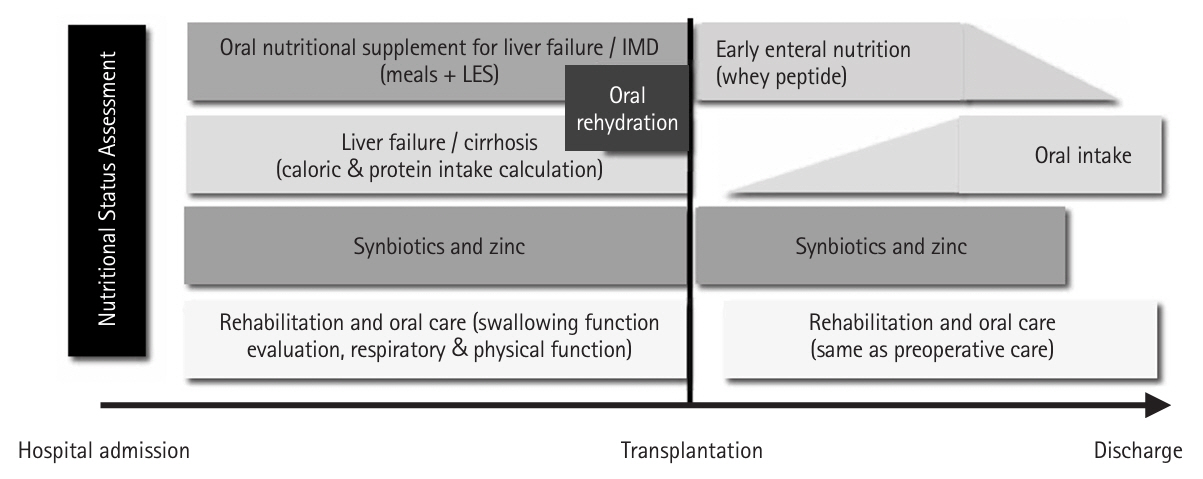
Based on these findings, we established a customized liver transplant perioperative nutritional rehabilitation therapy (hereafter referred to as “rehabilitation”), which includes nutritional assessment on admission and tailored interventions based on each patient’s nutritional status (
Fig. 1) [
5]. The following outlines the perioperative nutritional management protocol.
First, because the lack of preoperative branched-chain amino acids is an independent risk factor for post-transplant sepsis, oral amino acid products formulated for liver failure and enriched with branched-chain amino acids are administered between meals in the afternoon and before bedtime (late evening snack) from the time of admission. One week before surgery, immunomodulating nutritional supplements replace the standard oral amino acid formula for liver failure. The diet is then adjusted by a dietitian to avoid excessive calories and protein [
2].
Second, zinc supplementation should be initiated for patients with hypozincemia. While conventional zinc preparations do not rapidly increase blood zinc levels, administering a zinc acetate preparation containing a high concentration of zinc has been shown to significantly elevate blood zinc concentrations [
6].
Third, patients with decompensated cirrhosis are susceptible to disturbances in intestinal mucosal integrity due to portal hypertension. To prevent bacterial translocation, synbiotics—a combination of probiotics and prebiotics—are administered from the time of admission, as they have been shown to enhance intestinal immunity and reduce the incidence of post-transplant infections [
7].
Fourth, historically, patients were required to abstain from food and solids until dinner on the day before surgery and from liquids until lights out (approximately 10:00 pm). Currently, patients are permitted to consume solid foods until midnight on the day before surgery and fluids (such as oral rehydration solutions, water, or tea) until 6:00 am on the day of surgery. These preoperative nutritional interventions have significantly mitigated the decline in lymphocyte count and rise in C-reactive protein, while also significantly increasing prealbumin and zinc levels [
8].
Fifth, early initiation of enteral nutrition is central to postoperative management. At Kyoto University, an enterostomy tube was placed through the upper jejunum during liver transplantation, and enteral nutrition commenced within 24 hours after surgery. This regimen includes immunomodulating supplements containing whey peptides [
9], which possess anti-inflammatory and antioxidant properties. Notably, the incidence of postoperative bacteremia was significantly lower in the whey peptide immunomodulating nutrition group compared to the conventional digestive-form nutrition group [
5,
10].
Sixth, liver transplant recipients are susceptible to intestinal edema and paralysis resulting from factors such as portal hypertension, hypoalbuminemia, prolonged surgery, and extensive intraoperative fluid infusion. Consequently, the initiation of oral or enteral nutrition may be delayed or insufficient. We conducted a multicenter, double-blind, randomized, comparative study at 14 major liver transplant centers in Japan to assess the efficacy of postoperative Daiken-Chutou in enhancing gastrointestinal motility [
11]. Based on these findings, Daiken-chu-tang (15 g, minimum three doses) is administered via an enteral tube, particularly for patients experiencing intestinal dysmotility.
Seventh, postoperatively, patients continue to receive enteral or oral synbiotics until they can maintain adequate oral intake. Additionally, if serum zinc levels remain low, zinc acetate supplementation is administered.
Significance of sarcopenia in liver transplantation
Sarcopenia, defined as “loss of muscle mass, muscle strength, or physical function [
12,
13],” is classified into primary and secondary forms. Primary sarcopenia is related to aging, while secondary sarcopenia is associated with factors such as reduced physical activity (disuse), poor nutrition, organ failure, invasive procedures, tumors, and other diseases. Liver transplant recipients typically experience secondary sarcopenia due to decreased activity resulting from edema and ascites as well as poor nutritional status and liver failure.
Skeletal muscle mass can be assessed through whole-body or limb/trunk analysis, or via cross-sectional measurements of the trunk (often at the level of the third lumbar vertebra) using computed tomography (CT) or magnetic resonance imaging. We investigated the correlation between skeletal muscle mass determined by CT and that obtained by body composition analysis before liver transplantation in both donors and recipients. We found a strong correlation for donors (r=0.737) and recipients (r=0.682) [
14]. Given the high correlation between these two methods, the choice of measurement should depend on the availability of CT and body composition analysis equipment at each institution.
In our study, 38% of liver transplant recipients exhibited sarcopenia (low skeletal muscle mass), and post-transplant survival was significantly worse in patients with preoperative sarcopenia compared to those with higher skeletal muscle mass [
15]. Preoperative sarcopenia was identified as an independent risk factor for post-transplant mortality. Additionally, we examined the relationship between preoperative respiratory function and skeletal muscle mass, finding that lower muscle mass was associated with poorer respiratory function, including reduced lung capacity and lower expiratory volumes [
16]. We believe that preoperative rehabilitation is crucial for early postoperative recovery; accordingly, we have actively implemented preoperative rehabilitation programs that include respiratory muscle training, resistance exercises, and aerobic exercise using an ergometer alongside nutritional therapy [
17]. Thus, the combination of preoperative rehabilitation and nutritional therapy is vital in liver transplantation.
Beyond muscle mass, we also examined muscle quality and visceral fat obesity. We were the first to report that poor muscle quality and visceral fat obesity are independent adverse prognostic factors following liver transplantation [
18,
19].
Significance of perioperative nutritional therapy in liver transplantation
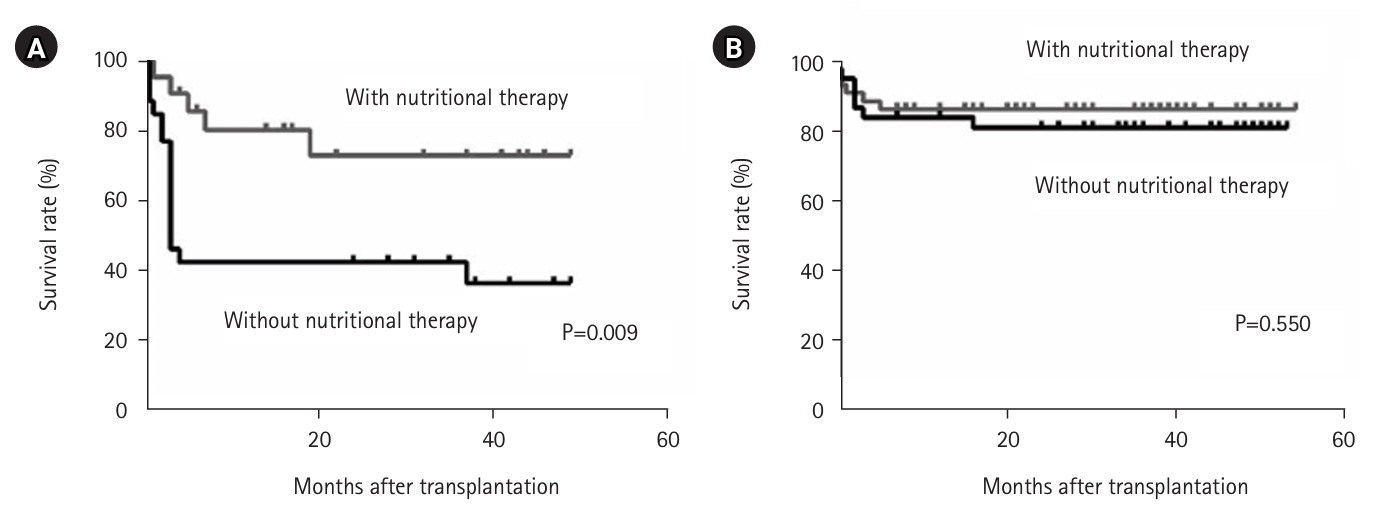
We examined the impact of perioperative nutritional therapy in patients with differing preoperative skeletal muscle mass. In patients with low skeletal muscle mass, postoperative survival was significantly improved by perioperative nutritional therapy (P=0.009) (
Fig. 2A) [
15]. In contrast, among patients with high skeletal muscle mass, the benefits of nutritional therapy on survival were minimal (
Fig. 2B) [
16]. Considering limited manpower and resources, we developed a treatment strategy focused on nutritional assessment at admission—including body composition analysis—with targeted nutritional intervention for patients with sarcopenia to improve short-term outcomes, further enhanced by preoperative rehabilitative intervention.
New indications for liver transplantation based on body composition
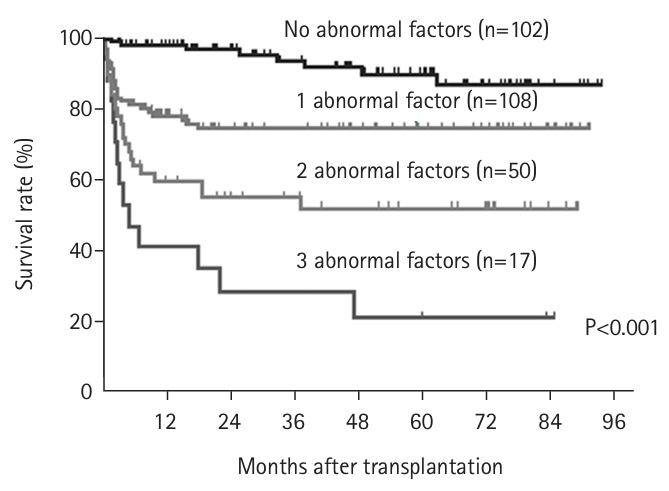
We investigated the prognostic impact of body composition (including skeletal muscle mass, muscle quality, and the visceral fat/subcutaneous fat ratio) in 277 patients who underwent living donor liver transplantation at Kyoto University between 2008 and July 2016. Preoperative simple CT at the L3 level was used to assess body composition. Using cutoff values derived from living liver transplant donors, we examined the prognostic significance of low skeletal muscle mass, poor muscle quality, and visceral fat obesity. We found that each abnormality was associated with poor prognosis and served as an independent risk factor [
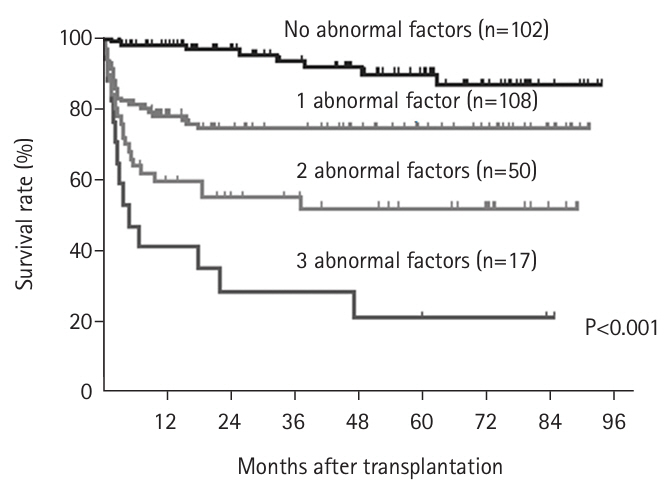
20]. Subsequently, we evaluated the impact of the number of abnormal factors on post-transplant survival. The 1-year survival rates were distinctly stratified according to the number of abnormalities: 98% with no abnormal factors, 78% with one, 60% with two, and 41% with three (P<0.001) (
Fig. 3) [
20]. To further elucidate these findings, we examined the relationship between the number of abnormal body composition factors and the incidence of postoperative bacteremia. We discovered that the incidence of bacteremia increased significantly with the number of abnormal factors [
21]. Moreover, the mortality rate for patients who developed bacteremia was 12% in the group with no abnormalities, escalating to 87% in the group with three abnormalities. In summary, preoperative abnormalities in body composition in living donor liver transplant recipients not only heighten the risk of postoperative bacteremia but also correlate with a high mortality rate once bacteremia develops. This suggests that preoperative body composition is closely linked to infectious complications, possibly due to decreased myokine production and increased adipokine levels that reduce immunocompetence.
Based on these results, since October 2016 we have recommended that patients with one or two abnormal body composition factors undergo aggressive perioperative nutritional rehabilitation to improve short-term outcomes after transplantation. Conversely, patients with three abnormal factors are advised to consider brain-dead liver transplantation with a larger liver and to receive nutritional rehabilitation during the waiting period, as they are deemed difficult to rescue with living donor liver transplantation. We established a new indication for living donor liver transplantation. We established a new indication for living donor liver transplantation and initiated its implementation. The 1-year survival rates of patients with 0, 1, 2, and 3 abnormalities were significantly stratified at 98%, 78%, 60%, and 41%, respectively (P<0.001) (
Fig. 3) [
20]. To clarify these results, we compared the 1-year survival rates in patients with body composition abnormalities to those with hepatic abnormalities. Further analysis revealed that the incidence of postoperative bacteremia increased significantly with the number of abnormal body composition factors [
21]. Furthermore, the mortality rate among patients with bacteremia was 12% in the group with no abnormalities, rising to 87% in those with three abnormalities. In other words, preoperative body composition abnormalities in living donor liver transplant recipients not only heighten the risk of postoperative bacteremia but also lead to a high mortality rate once bacteremia develops. Therefore, preoperative body composition and infection are closely interrelated.
As a result of these comprehensive interventions, an exceptional 1-year survival rate of 99% was achieved after liver transplantation [
22]. Advances in perioperative management, including nutritional management, and the introduction of new surgical techniques [
23,
24] contributed to the improvement of transplantation outcomes.
Conclusion
Numerous clinical studies were initiated in response to needs identified in clinical practice. Based on these findings, we established a policy of “new indications for liver transplantation and perioperative nutrition and rehabilitation intervention based on body composition” and prospectively validated its effectiveness. Consequently, we achieved an outstanding 1-year survival rate of 99% after liver transplantation. The importance of perioperative nutritional management is now being recognized in other organ transplants, and this study represents a breakthrough in improving transplantation outcomes.
Authors’ contribution
All work was done by Toshimi Kaido.
Conflict of interest
The author of this manuscript has no conflicts of interest to disclose.
Funding
None.
Data availability
Not applicable.
Acknowledgments
None.
Supplementary materials
None.
Fig. 1.Customized perioperative nutritional rehabilitation therapy for liver transplant patients. IMD, immunomodulatory nutritional supplement; LES, late evening snack.
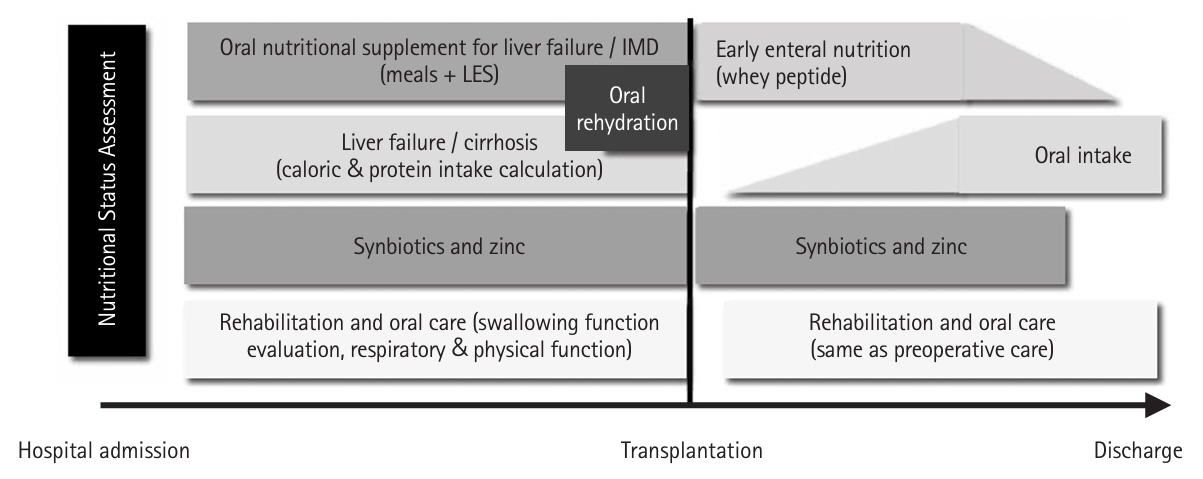
Fig. 2.Survival rate after liver transplantation with and without perioperative nutritional therapy in patients with low preoperative skeletal muscle mass (A) and high skeletal muscle mass (B) (log-rank test).
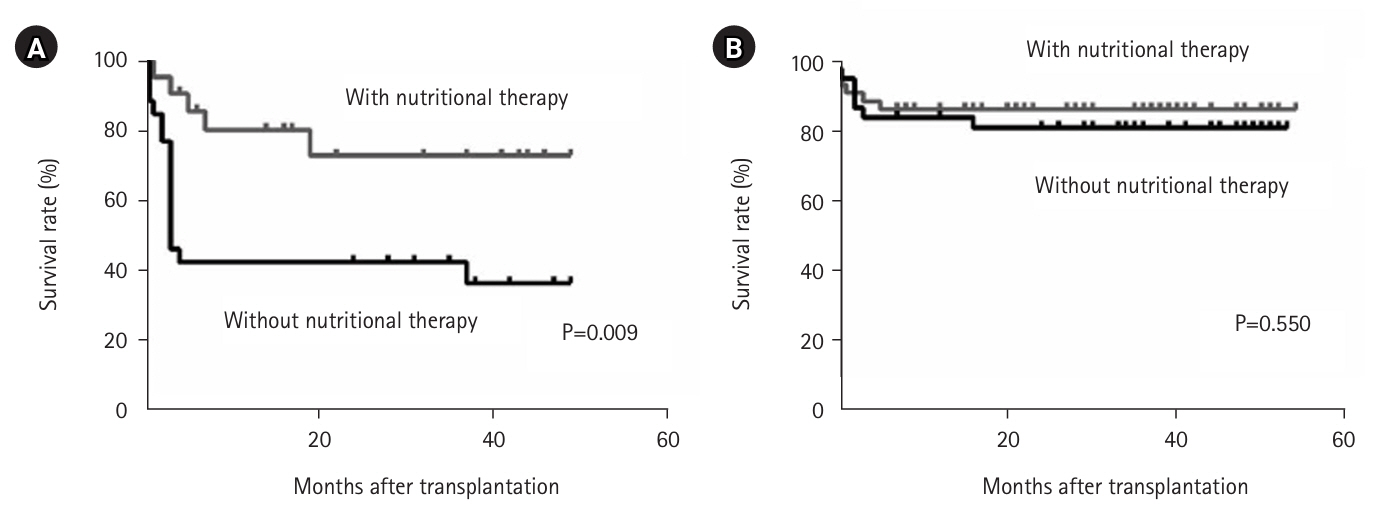
Fig. 3.Number of factors contributing to abnormal preoperative body composition (skeletal muscle mass, muscle quality, visceral fat obesity) and survival rate after liver transplantation (log-rank test).
References
- 1. Kaido T, Egawa H, Tsuji H, Ashihara E, Maekawa T, Uemoto S. In-hospital mortality in adult recipients of living donor liver transplantation: experience of 576 consecutive cases at a single center. Liver Transpl 2009;15:1420-5.ArticlePubMedPDF
- 2. Kaido T, Mori A, Ogura Y, Ogawa K, Hata K, Yoshizawa A, et al. Pre- and perioperative factors affecting infection after living donor liver transplantation. Nutrition 2012;28:1104-8.ArticlePubMed
- 3. Hammad A, Kaido T, Yagi S, Okajima H, Uemoto S. Characteristics of nutritional status and the effect of pre-transplant branched-chain amino acid administration in patients undergoing living donor liver transplantation. J Clin Exp Transplant 2016;1:1000101.Article
- 4. Hammad A, Kaido T, Ogawa K, Fujimoto Y, Tomiyama K, Mori A, et al. Perioperative changes in nutritional parameters and impact of graft size in patients undergoing adult living donor liver transplantation. Liver Transpl 2014;20:1486-96.ArticlePubMed
- 5. Kaido T, Ogura Y, Ogawa K, Hata K, Yoshizawa A, Yagi S, et al. Effects of post-transplant enteral nutrition with an immunomodulating diet containing hydrolyzed whey peptide after liver transplantation. World J Surg 2012;36:1666-71.ArticlePubMedPDF
- 6. Kaido T, Toshimi K. Significance of perioperative nutritional management in liver transplantation. Clin J Surg 2019;81:644-8.Article
- 7. Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation: a randomized, double-blind trial. Am J Transplant 2005;5:125-30.ArticlePubMed
- 8. Tamai Y, Kaido T, Hata K, Hei K, Uemoto S, Inagaki N, et al. Impact of preoperative nutritional therapy in patients undergoing liver transplantation. Jomyaku Keicho Eiyo 2012;27:1229-37.Article
- 9. Jobara K, Kaido T, Hori T, Iwaisako K, Endo K, Uchida Y, et al. Whey-hydrolyzed peptide-enriched immunomodulating diet prevents progression of liver cirrhosis in rats. Nutrition 2014;30:1195-207.ArticlePubMed
- 10. Kamo N, Kaido T, Hamaguchi Y, Uozumi R, Okumura S, Kobayashi A, et al. Impact of enteral nutrition with an immunomodulating diet enriched with hydrolyzed whey peptide on infection after liver transplantation. World J Surg 2018;42:3715-25.ArticlePubMedPDF
- 11. Kaido T, Shinoda M, Inomata Y, Yagi T, Akamatsu N, Takada Y, et al. Effect of herbal medicine daikenchuto on oral and enteral caloric intake after liver transplantation: a multicenter, randomized controlled trial. Nutrition 2018;54:68-75.ArticlePubMed
- 12. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23.ArticlePubMedPMC
- 13. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101.ArticlePubMed
- 14. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5.ArticlePubMed
- 15. Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549-56.ArticlePubMed
- 16. Shirai H, Kaido T, Hamaguchi Y, Yao S, Kobayashi A, Okumura S, et al. Preoperative low muscle mass has a strong negative effect on pulmonary function in patients undergoing living donor liver transplantation. Nutrition 2018;45:1-10.ArticlePubMed
- 17. Yoshioka Y, Oshima Y, Hamada R, Shimamura N, Sato S, Kaido T, et al. Effect and safety of rehabilitation program before living-donor liver transplantation. Jpn J Transpl 2019;54:211-6.Article
- 18. Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014;20:1413-9.ArticlePubMedPDF
- 19. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 2017;101:565-74.ArticlePubMed
- 20. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao S, et al. Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J Cachexia Sarcopenia Muscle 2018;9:246-54.ArticlePubMedPMCPDF
- 21. Kamo N, Kaido T, Miyachi Y, Iwamura S, Yao S, Shirai H, et al. Preoperative abnormal body composition is closely related to bacteremia after living donor liver transplantation. Nutrition 2020;77:110798.ArticlePubMed
- 22. Kaido T. Recent evolution of living donor liver transplantation at Kyoto University: how to achieve a one-year overall survival rate of 99%? Hepatobiliary Pancreat Dis Int 2020;19:328-33.ArticlePubMed
- 23. Yao S, Kaido T, Uozumi R, Yagi S, Miyachi Y, Fukumitsu K, et al. Is Portal venous pressure modulation still indicated for all recipients in living donor liver transplantation? Liver Transpl 2018;24:1578-88.ArticlePubMedPDF
- 24. Yao S, Kaido T, Yagi S, Uozumi R, Iwamura S, Miyachi Y, et al. Impact of imbalanced graft-to-spleen volume ratio on outcomes following living donor liver transplantation in an era when simultaneous splenectomy is not necessarily indicated. Am J Transplant 2019;19:2783-94.ArticlePubMed






 KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 ePub Link
ePub Link Cite
Cite

