Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > J Clin Nutr > Volume 9(1); 2017 > Article
- Original Article Influence of Fish Oil-Containing Lipid Emulsions on Parenteral Nutrition-Associated Liver Disease in Neonates
- Jeong-A Park1, Ji-Eun Park1,2, Min-Jae Jeong1,2, Jae-Song Kim1, Eun-Sun Son1, Ho-Seon Eun2,3
- 신생아 환자에서 지방 유제 사용 시 어유 함유 여부에 따른 Parenteral Nutrition-Associated Liver Disease 발생 정도 분석
- 박정아1, 박지은1,2, 정민재1,2, 김재송1, 손은선1, 은호선2,3
-
Journal of the Korean Society for Parenteral and Enteral Nutrition 2017;9(1):21-29.
DOI: https://doi.org/10.15747/jcn.2017.9.1.21
Published online: June 30, 2017
Department of Pharmacy, Severance Hospital, Seoul, Korea
Pediatric Nutrition Support Team, Severance Hospital, Seoul, Korea
Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea
- Correspondence to Jeong-A Park Department of Pharmacy, Severance Hospital, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-6896, Fax: +82-2-312-5732, E-mail: japark1216@yuhs.ac
Copyright: © Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 869 Views
- 1 Download
Abstract
-
Purpose: This study is a comparative evaluation of the incidence of parenteral nutrition-associated liver disease (PNALD) when administering intravenous fat emulsions containing fish oil.
-
Methods: The medical records of patients who were in the neonatal intensive care unit at Severance Hospital from January, 2012 to December 2015, were reviewed retrospectively. Patients who were administered either soybean oil (SO) or SMOF (containing soybean oil, medium chain triglycerides, olive oil, and fish oil) more than 14 days were included. The patients were excluded if they were administered both agents or had underlying hepatic disease. An increase in bilirubin to 2 mg/dL was defined as PNALD.
-
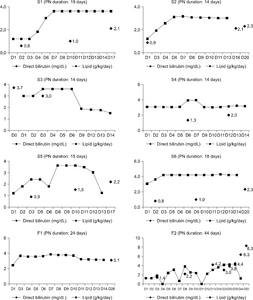
Results: PNALD occurred in only 8 out of a total of 77 patients: 6 out of 31 (19.4%) in the SO group and 2 out of 46 (4.3%) in the SMOF group (P=0.055). The number of patients, whose lab values, such as direct bilirubin, total bilirubin, asparate aminotransferase (AST), alanine amino-transferase, gamma-glutamyl transpeptidase, C-reactive protein, serum triglyceride, and alkaline phosphate, exceeded the normal range, were similar in both groups. The gestational age, birth body weight, and APGAR score at 1 min and 5 min were significantly higher in the SO group and the PN duration was significantly long in the SMOF group. Considering only term infants, there were no significant differences in baseline characteristics and incidence of PNALD. The number of patients whose AST exceeded the normal range was significantly higher in the SO group (P=0.034).
-
Conclusion: The incidence of PNALD was similar in both groups. On the other hand, considering the tendency, there was a high correlation between the type of lipid emulsion and an increased direct bilirubin level in the SO group.
서론
대상 및 방법
1) 대상자 선정기준
2) 대상자 제외기준
3) 데이터 수집 항목
결과
1) 출생 시 특성
| Characteristic | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 16 (51.6) | 23 (50.0) | |
| Female | 15 (48.4) | 23 (50.0) | |
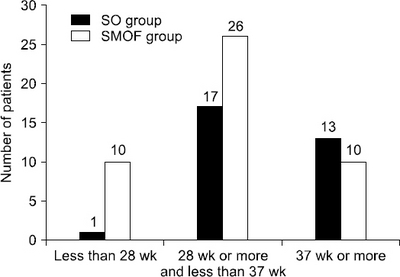
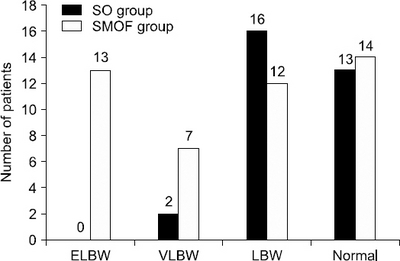
| GA (wk) | 35.69±3.13 | 32.37±5.02 | 0.001 |
| GA<28 | 1 (3.2) | 10 (21.7) | 0.023 |
| 28≤GA<37 | 17 (54.8) | 26 (56.5) | 0.884 |
| GA≥37 | 13 (41.9) | 10 (21.7) | 0.058 |
| Birth weight (kg) | 2.35±0.65 | 1.80±0.93 | 0.003 |
| APGAR scorea | |||
| 1 min | 5.13±1.94 | 3.84±2.40 | 0.016 |
| 5 min | 6.77±1.54 | 5.63±2.20 | 0.010 |
Values are presented as number (%) or mean±standard deviation. SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; GA = gestational age.
aAPGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color
2) TPN 투여기간
| Duration | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| TPN duration (d) | 19.42±8.31 | 25.85±11.49 | 0.006 |
| TPN duration depending on combination with ENa (n=49) | |||
| NPO+PN (d) | 6.96±5.30 | 4.58±4.76 | 0.107 |
| EN+PN (d) | 12.57±11.47 | 23.46±14.18 | 0.005 |
| EN+PN/total TPN duration ratio (%) | 59.24±29.33 | 80.10±27.87 | 0.014 |
Values are presented as mean±standard deviation.
TPN = total parenteral nutrition; SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; NPO = nothing per oral; EN = enteral nutrition; PN = parenteral nutrition.
aEvaluation of patients who only can be checked about feeding.
3) PNALD 관련 질병
| Underlying diseases | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| PDA | 13 (41.9) | 18 (39.1) | 0.894 |
| NEC | 0 (0) | 1 (2.2) | 0.401 |
| Sepsis | 4 (12.9) | 10 (21.7) | 0.296 |
| TPN supply | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| Lipid (g/kg/d) | 2.83±0.83 | 3.06±1.09 | <0.05 |
| GIR (mg/kg/min) | 10.17±9.74 | 9.32±8.43 | <0.05 |
| Amino acid (g/kg/d) | 2.48±0.72 | 2.61±0.90 | <0.05 |
| Term infants | SO group (n=13) | SMOF group (n=10) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 6 (46.2) | 4 (40.0) | |
| Female | 7 (53.8) | 6 (60.0) | |
| Birth weight (kg) | 2.92±0.42 | 3.01±0.33 | 0.574 |
| APGAR scorea | |||
| 1 min | 6.23±1.54 | 6.50±2.07 | 0.736 |
| 5 min | 7.46±1.20 | 7.75±1.58 | 0.640 |
| TPN duration (d) | 24.64±13.18 | 22.30±8.95 | 0.631 |
| TPN duration depending on combination with ENb (n=13) | |||
| NPO+PN (d) | 9.00±6.57 | 8.60±6.91 | 0.918 |
| EN+PN (d) | 16.13±18.22 | 14.80±15.42 | 0.895 |
| PDA | 8 (61.5) | 4 (40.0) | 0.305 |
| NEC | 0 (0) | 0 (0) | - |
| Sepsis | 2 (15.4) | 0 (0) | 0.194 |
Values are presented as number (%) or mean±standard deviation.
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; TPN = total parenteral nutrition; NPO = nothing per oral; PN = parenteral nutrition; EN = enteral nutrition; PDA = patent ductus arteriosus; NEC = necrotic enterocolitis.
aAPGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color.
bEvaluation of patients who only can be checked about feeding.
고찰
| Characteristic | SO group (n=6) | SMOF group (n=2) | P-value |
|---|---|---|---|
| Gestational age (wk) | 37.1±2.65 | 32.85±8.84 | 0.622 |
| Birth weight (kg) | 2.63±0.52 | 1.92±1.35 | 0.589 |
| APGAR scorea | |||
| 1 min | 5.50±2.35 | 2.00±2.83 | 0.128 |
| 5 min | 6.50±2.81 | 4.00±2.83 | 0.318 |
결론
Appendix
- 1. Nandivada P, Chang MI, Potemkin AK, Carlson SJ, Cowan E, Oʼloughlin AA, et al. The natural history of cirrhosis from parenteral nutrition-associated liver disease after resolution of cholestasis with parenteral fish oil therapy. Ann Surg 2015;261(1):172-9. ArticlePubMed
- 2. Wales PW, Allen N, Worthington P, George D, Compher C. American Society for Parenteral, and Enteral Nutrition, Teitelbaum D. A.S.P.E.N. clinical guidelines:support of pediatric patients with intestinal failure at risk of parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr 2014;38(5):538-57. ArticlePubMed
- 3. Rangel SJ, Calkins CM, Cowles RA, Barnhart DC, Huang EY, Abdullah F, et al. Parenteral nutrition-associated cholestasis:an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 2012;47(1):225-40. ArticlePubMed
- 4. Park HW, Lee NM, Kim JH, Kim KS, Kim SN. Parenteral fish oil-containing lipid emulsions may reverse parenteral nutrition- associated cholestasis in neonates:a systematic review and meta-analysis. J Nutr 2015;145(2):277-83. ArticlePubMed
- 5. Deshpande G, Simmer K. Lipids for parenteral nutrition in neonates. Curr Opin Clin Nutr Metab Care 2011;14(2):145-50. ArticlePubMed
- 6. Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellöf M, et al. ESPGHAN Committee on Nutrition Position Paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children:a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr 2016;62(5):776-92. ArticlePubMed
- 7. Krohn K, Koletzko B. Parenteral lipid emulsions in paediatrics. Curr Opin Clin Nutr Metab Care 2006;9(3):319-23. ArticlePubMed
- 8. Iyer KR, Spitz L, Clayton P. BAPS prize lecture:new insight into mechanisms of parenteral nutrition-associated cholestasis:role of plant sterols. British Association of Paediatric Surgeons. J Pediatr Surg 1998;33(1):1-6. ArticlePubMed
- 9. Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition- associated cholestatic liver disease. Gastroenterology 1993;105(6):1806-13. ArticlePubMed
- 10. Nikkilä K, Nissinen MJ, Gylling H, Isoniemi H, Miettinen TA. Serum sterols in patients with primary biliary cirrhosis and acute liver failure before and after liver transplantation. J Hepatol 2008;49(6):936-45. ArticlePubMed
- 11. Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 2009;250(3):395-402. ArticlePubMed
- 12. Yoon JH. Parenteral nutrition support in preterm infants. J Clin Nutr 2014;6(2):51-5. Article
- 13. Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, Antoniadi M, et al. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/ω-3- PUFA-containing or soybean-based lipid emulsions. Nutr Clin Pract 2012;27(6):817-24. ArticlePubMedPDF
- 14. Savini S, D’Ascenzo R, Biagetti C, Serpentini G, Pompilio A, Bartoli A, et al. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition:a randomized clinical trial. Am J Clin Nutr 2013;98(2):312-8. ArticlePubMed
- 15. Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil:a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr 2010;51(4):514-21. ArticlePubMed
- 16. Beken S, Dilli D, Fettah ND, Kabataş EU, Zenciroğlu A, Okumuş N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants:a randomized controlled trial. Early Hum Dev 2014;90(1):27-31. ArticlePubMed
- 17. Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, van den Akker CH, van Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pediatr Gastroenterol Nutr 2014;58(4):417-27. ArticlePubMed
- 18. Dounousi E, Zikou X, Koulouras V, Katopodis K. Metabolic acidosis during parenteral nutrition:Pathophysiological mechanisms. Indian J Crit Care Med 2015;19(5):270-4. ArticlePubMedPMC
References
Figure & Data
REFERENCES
Citations

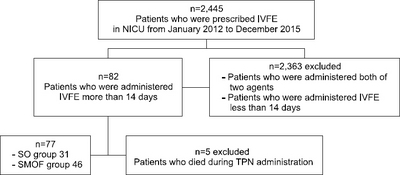
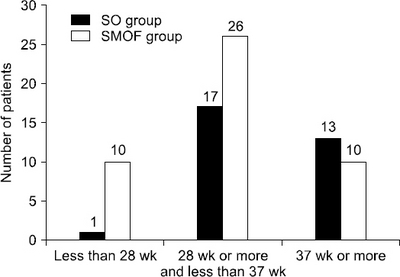
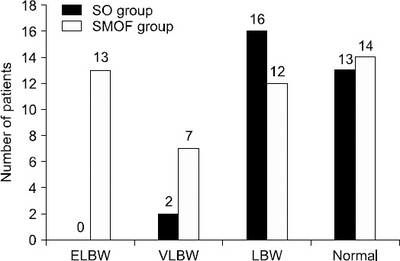
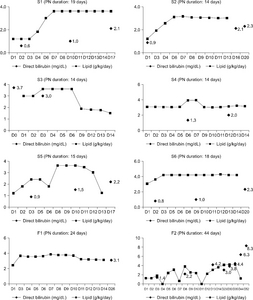
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Birth characteristics (n=77)
| Characteristic | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 16 (51.6) | 23 (50.0) | |
| Female | 15 (48.4) | 23 (50.0) | |
| GA (wk) | 35.69±3.13 | 32.37±5.02 | 0.001 |
| GA<28 | 1 (3.2) | 10 (21.7) | 0.023 |
| 28≤GA<37 | 17 (54.8) | 26 (56.5) | 0.884 |
| GA≥37 | 13 (41.9) | 10 (21.7) | 0.058 |
| Birth weight (kg) | 2.35±0.65 | 1.80±0.93 | 0.003 |
| APGAR score |
|||
| 1 min | 5.13±1.94 | 3.84±2.40 | 0.016 |
| 5 min | 6.77±1.54 | 5.63±2.20 | 0.010 |
Values are presented as number (%) or mean±standard deviation. SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; GA = gestational age.
aAPGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color
TPN duration (n=77)
| Duration | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| TPN duration (d) | 19.42±8.31 | 25.85±11.49 | 0.006 |
| TPN duration depending on combination with EN |
|||
| NPO+PN (d) | 6.96±5.30 | 4.58±4.76 | 0.107 |
| EN+PN (d) | 12.57±11.47 | 23.46±14.18 | 0.005 |
| EN+PN/total TPN duration ratio (%) | 59.24±29.33 | 80.10±27.87 | 0.014 |
Values are presented as mean±standard deviation.
TPN = total parenteral nutrition; SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; NPO = nothing per oral; EN = enteral nutrition; PN = parenteral nutrition.
aEvaluation of patients who only can be checked about feeding.
Underlying diseases related to parenteral nutrition-associated liver disease (n=77)
| Underlying diseases | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| PDA | 13 (41.9) | 18 (39.1) | 0.894 |
| NEC | 0 (0) | 1 (2.2) | 0.401 |
| Sepsis | 4 (12.9) | 10 (21.7) | 0.296 |
Values are presented as number (%).
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; PDA = patent ductus arteriosus; NEC = necrotic enterocolitis.
Laboratory test (n=77)
| Laboratory test | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| The number of patients who observed on direct bilirubin ≥2 mg/dL | 6 (19.4) | 2 (4.3) | 0.055 |
| Lab value exceeded normal range | |||
| Direct bilirubin | 24 (77.4) | 39 (84.8) | 0.207 |
| Total bilirubin | 28 (90.3) | 41 (89.1) | 1.000 |
| AST | 20 (64.5) | 21 (45.7) | 0.104 |
| ALT | 4 (12.9) | 2 (4.3) | 0.213 |
| Triglyceride | 4 (12.9) | 7 (15.2) | 1.000 |
| ALP | 23 (74.2) | 39 (84.8) | 0.250 |
| CRP | 4 (12.9) | 5 (10.9) | 1.000 |
| γ-GT | 4 (12.9) | 5 (10.9) | 1.000 |
| Diagnosed with metabolic acidosis | 0 (0) | 1 (2.2) | 0.409 |
Values are presented as number (%).
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; AST = asparate aminotransferase; ALT = alanine amino-transferase; ALP = alkaline phosphatase; CRP = C-reactive protein; γ-GT = gamma-glutamyl transpeptidase.
TPN supply
| TPN supply | SO group (n=31) | SMOF group (n=46) | P-value |
|---|---|---|---|
| Lipid (g/kg/d) | 2.83±0.83 | 3.06±1.09 | <0.05 |
| GIR (mg/kg/min) | 10.17±9.74 | 9.32±8.43 | <0.05 |
| Amino acid (g/kg/d) | 2.48±0.72 | 2.61±0.90 | <0.05 |
Values are presented as mean±standard deviation.
TPN = total parenteral nutrition; SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; GIR = glucose infusion rate.
Data of term infants (n=23)
| Term infants | SO group (n=13) | SMOF group (n=10) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 6 (46.2) | 4 (40.0) | |
| Female | 7 (53.8) | 6 (60.0) | |
| Birth weight (kg) | 2.92±0.42 | 3.01±0.33 | 0.574 |
| APGAR score |
|||
| 1 min | 6.23±1.54 | 6.50±2.07 | 0.736 |
| 5 min | 7.46±1.20 | 7.75±1.58 | 0.640 |
| TPN duration (d) | 24.64±13.18 | 22.30±8.95 | 0.631 |
| TPN duration depending on combination with EN |
|||
| NPO+PN (d) | 9.00±6.57 | 8.60±6.91 | 0.918 |
| EN+PN (d) | 16.13±18.22 | 14.80±15.42 | 0.895 |
| PDA | 8 (61.5) | 4 (40.0) | 0.305 |
| NEC | 0 (0) | 0 (0) | - |
| Sepsis | 2 (15.4) | 0 (0) | 0.194 |
Values are presented as number (%) or mean±standard deviation.
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; TPN = total parenteral nutrition; NPO = nothing per oral; PN = parenteral nutrition; EN = enteral nutrition; PDA = patent ductus arteriosus; NEC = necrotic enterocolitis.
aAPGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color.
bEvaluation of patients who only can be checked about feeding.
Laboratory test_term infants (n=23)
| Laboratory test | SO group (n=13) | SMOF group (n=10) | P-value |
|---|---|---|---|
| Observed on direct bilirubin≥2 mg/dL | 4 (30.8) | 1 (10.0) | 0.231 |
| Lab value exceeded normal range | |||
| Direct bilirubin | 9 (69.2) | 5 (50.0) | 0.349 |
| Total bilirubin | 11 (84.6) | 7 (70.0) | 0.400 |
| AST | 13 (100.0) | 7 (70.0) | 0.034 |
| ALT | 3 (23.1) | 1 (10.0) | 0.412 |
| Triglyceride | 2 (15.4) | 1 (10.0) | 0.704 |
| ALP | 9 (69.2) | 7 (70.0) | 0.968 |
| CRP | 4 (30.8) | 4 (40.0) | 0.645 |
| γ-GT | 4 (30.8) | 3 (30.0) | 0.968 |
| Diagnosed with metabolic acidosis | 0 (0) | 1 (10.0) | 0.244 |
Values are presented as number (%).
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; AST = asparate aminotransferase; ALT = alanine amino-transferase; ALP = alkaline phosphatase; CRP = C-reactive protein; γ-GT = gamma-glutamyl transpeptidase.
Birth characteristics of patients diagnosed parenteral nutrition-associated liver disease (n=8)
| Characteristic | SO group (n=6) | SMOF group (n=2) | P-value |
|---|---|---|---|
| Gestational age (wk) | 37.1±2.65 | 32.85±8.84 | 0.622 |
| Birth weight (kg) | 2.63±0.52 | 1.92±1.35 | 0.589 |
| APGAR score |
|||
| 1 min | 5.50±2.35 | 2.00±2.83 | 0.128 |
| 5 min | 6.50±2.81 | 4.00±2.83 | 0.318 |
Values are presented as mean±standard deviation.
SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil.
aAPGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color.
Values are presented as number (%) or mean±standard deviation. SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; GA = gestational age. APGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color
Values are presented as mean±standard deviation. TPN = total parenteral nutrition; SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; NPO = nothing per oral; EN = enteral nutrition; PN = parenteral nutrition. Evaluation of patients who only can be checked about feeding.
Values are presented as number (%). SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; PDA = patent ductus arteriosus; NEC = necrotic enterocolitis.
Values are presented as number (%). SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; AST = asparate aminotransferase; ALT = alanine amino-transferase; ALP = alkaline phosphatase; CRP = C-reactive protein; γ-GT = gamma-glutamyl transpeptidase.
Values are presented as mean±standard deviation. TPN = total parenteral nutrition; SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; GIR = glucose infusion rate.
Values are presented as number (%) or mean±standard deviation. SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; TPN = total parenteral nutrition; NPO = nothing per oral; PN = parenteral nutrition; EN = enteral nutrition; PDA = patent ductus arteriosus; NEC = necrotic enterocolitis. APGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color. Evaluation of patients who only can be checked about feeding.
Values are presented as number (%). SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil; AST = asparate aminotransferase; ALT = alanine amino-transferase; ALP = alkaline phosphatase; CRP = C-reactive protein; γ-GT = gamma-glutamyl transpeptidase.
Values are presented as mean±standard deviation. SO = soybean oil; SMOF = soybean oil, medium-chain triglycerides, olive oil, fish oil. APGAR score: Method to quickly summarize the health of neonate by scoring of breathing effort, heart rate, muscle tone, reflexes, skin color.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite

