Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 11(2); 2020 > Article
- Review Article Muscle Protein Metabolism in Critically Illness
-
Min Chang Kang, M.D.

-
Surgical Metabolism and Nutrition 2020;11(2):35-39.
DOI: https://doi.org/10.18858/smn.2020.11.2.35
Published online: December 30, 2020
Division of General Surgery, Soonchunhyang University Seoul Hospital, Seoul, Korea
- Corresponding author : Min Chang Kang E-mail stserver@naver.com ORCID https://orcid.org/0000-0002-0834-6060
Copyright © 2020 The Korean Society of Surgical Metabolism and Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,772 Views
- 34 Download
- 6 Crossref
Abstract
- Most patients experience a considerable amount of muscle wasting during critical care. A decrease in muscle mass causes weakness which inevitably leads to delayed recovery. Since muscle also plays an important role in protein metabolism, metabolic instability increases as muscle mass decreases. Accordingly, various treatments have been attempted to maintain muscle mass and function in critically ill patients; however, it is still difficult to prevent muscle loss. It is known that muscle wasting in critical illness is primarily due to increased muscle protein breakdown rather than a decrease in muscle protein synthesis. Nutritional therapy and rehabilitation are fundamentally important, but additional anabolic agents may be needed to overcome anabolic resistance. In this review, we will learn about muscle protein metabolism in critically ill patients and how various treatments affect muscle protein metabolism.
INTRODUCTION
WHOLE-BODY PROTEIN TURNOVER
NORMAL MUSCLE PROTEIN BALANCE
STABLE ISOTOPE TRACER METHOD
CRITICAL ILLNESS
CONCLUSION
| MPS | MPB | NPB | |
|---|---|---|---|
| Normal (post-prandial) | ++ | + | + |
| Normal (fasting) | + | ++ | - |
| Catabolic state (fasting) | +++ | ++++++ | --- |
| Catabolic state (protein supply) | ++++ | ++++++ | -- |
| Catabolic state (rehabilitation) | ? | ? | - |
- 1. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475-82. ArticlePubMed
- 2. Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, et al. Muscle loss: the new malnutrition challenge in clinical practice. Clin Nutr 2019;38:2113-20. ArticlePubMed
- 3. Wolfe RR. The 2017 sir David P Cuthbertson lecture. Amino acids and muscle protein metabolism in critical care. Clin Nutr 2018;37:1093-100. ArticlePubMed
- 4. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591-600. ArticlePubMed
- 5. Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr 2004;23:273-80. ArticlePubMed
- 6. Hickmann CE, Castanares-Zapatero D, Deldicque L, Van den Bergh P, Caty G, Robert A, et al. Impact of very early physical therapy during septic shock on skeletal muscle: a randomized controlled trial. Crit Care Med 2018;46:1436-43. ArticlePubMedPMC
- 7. De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859-67. ArticlePubMed
- 8. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931-41. ArticlePubMed
- 9. Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626-35. ArticlePubMed
- 10. Puthucheary Z, Denehy L. Chronic critical illness and muscle strength: an ill-defined field. Crit Care Med 2020;48:1699-701. ArticlePubMed
- 11. Attaix D, Boirie Y. In: Cano N, Barnoud D, Schneider SM, Vasson MP, Hasselmann M, Leverve X, editors. 2007. Métabolisme protéique. Traité de nutrition artificielle de l’adulte. 3rd ed. Springer; Paris: p. 75-92 French. Article
- 12. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. ArticlePubMed
- 13. Kim IY, Suh SH, Lee IK, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med 2016;48:e203. ArticlePubMedPMCPDF
- 14. Bodine SC. 2006;mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 38:1950-7. ArticlePubMed
- 15. Phillips SM. Protein requirements and supplementation in strength sports. Nutrition 2004;20:689-95. ArticlePubMed
- 16. Zhang XJ, Chinkes DL, Cox RA, Wolfe RR. The flow phase of wound metabolism is characterized by stimulated protein synthesis rather than cell proliferation. J Surg Res 2006;135:61-7. ArticlePubMed
- 17. Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 1987;205:288-94. ArticlePubMedPMC
- 18. Miller S, Chinkes D, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab 2004;287:E136-41. ArticlePubMed
- 19. Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab 2002;87:3378-84. ArticlePubMed
- 20. Tuvdendorj D, Chinkes DL, Zhang XJ, Ferrando AA, Elijah IE, Mlcak RP, et al. Adult patients are more catabolic than children during acute phase after burn injury: a retrospective analysis on muscle protein kinetics. Intensive Care Med 2011;37:1317-22. ArticlePubMedPMCPDF
- 21. Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab 2016;310:E73-80. ArticlePubMed
- 22. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250-8. ArticlePubMedPMC
- 23. Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res 2008;28:651-8. ArticlePubMedPMC
- 24. Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr 2013;32:309-13. ArticlePubMed
- 25. Liebau F, Sundström M, van Loon LJ, Wernerman J, Rooyackers O. Short-term amino acid infusion improves protein balance in critically ill patients. Crit Care 2015;19:106.ArticlePubMedPMCPDF
- 26. Phillips SM, Dickerson RN, Moore FA, Paddon-Jones D, Weijs PJ. Protein turnover and metabolism in the elderly intensive care unit patient. Nutr Clin Pract 2017;32(1_suppl):112S-20S. ArticlePubMedPDF
- 27. Dideriksen K, Reitelseder S, Agergaard J, Boesen AP, Aas SN, Raastad T, et al. 2020;Muscle protein breakdown is impaired during immobilization compared to during a subsequent retraining period in older men: no effect of anti-inflammatory medication. Pflugers Arch 472:281-92. ArticlePubMedPMCPDF
- 28. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 1996;270(4 Pt 1):E627-33. ArticlePubMed
- 29. Kayambu G, Boots R, Paratz J. 2013;Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med 41:1543-54. ArticlePubMed
- 30. Laurent H, Aubreton S, Richard R, Gorce Y, Caron E, Vallat A, et al. Systematic review of early exercise in intensive care: a qualitative approach. Anaesth Crit Care Pain Med 2016;35:133-49. ArticlePubMed
- 31. Supinski GS, Valentine EN, Netzel PF, Schroder EA, Wang L, Callahan LA. Does standard physical therapy increase quadriceps strength in chronically ventilated patients? A pilot study. Crit Care Med 2020;48:1595-603. ArticlePubMedPMC
- 32. Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 2012;95:1403-12. ArticlePubMedPMC
- 33. Heyland DK, Day A, Clarke GJ, Hough CT, Files DC, Mourtzakis M, et al. Nutrition and exercise in critical illness trial (NEXIS Trial): a protocol of a multicentred, randomised controlled trial of combined cycle ergometry and amino acid supplementation commenced early during critical illness. BMJ Open 2019;9:e027893. ArticlePubMedPMC
- 34. Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, et al. 2016;Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594:5209-22. ArticlePubMedPMCPDF
- 35. Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med 2001;29:1936-42. ArticlePubMed
- 36. Wolf SE, Thomas SJ, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg 2003;237:801-10. ArticlePubMedPMC
- 37. Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 1999;229:713-20. ArticlePubMedPMC
- 38. Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999;229:11-8. ArticlePubMedPMC
- 39. Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 2001;345:1223-9. ArticlePubMed
- 40. Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 2003;88:358-62. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

- Combined intravenous bolus amino acid supplementation and mobilization on early muscle loss in critically ill adults: A randomized controlled trial
Lizl Veldsman, Guy A. Richards, Daniel Nel, Tertius A. Kohn, Renée Blaauw
Journal of Parenteral and Enteral Nutrition.2026; 50(2): 170. CrossRef - Low protein content of plant-derived nutrition limits the protein transition in hospitalized patients: Results from an observational study
M.A. van Bree, B.C. Schouten, E.S. Wolters, M.R. Soeters, H.M. Kruizenga
Clinical Nutrition ESPEN.2025; 69: 311. CrossRef - Combined Protein, Probiotics, and Exercise Therapy for Sarcopenia: A Comprehensive Review
Ryuk Jun Kwon, Mohammad Al Mijan, Soo Min Son, Wanho Yoo, Taehwa Kim
Cells.2025; 14(17): 1375. CrossRef - Double-Edge Effects of Leucine on Cancer Cells
Burkitkan Akbay, Zhannur Omarova, Alexander Trofimov, Bayan Sailike, Orynbassar Karapina, Ferdinand Molnár, Tursonjan Tokay
Biomolecules.2024; 14(11): 1401. CrossRef - Compartmental analysis: a new approach to estimate protein breakdown and meal response in health and critical illness
Nicolaas E. P. Deutz, Mariëlle P. K. J. Engelen
Frontiers in Nutrition.2024;[Epub] CrossRef - Amino acid kinetics in the critically ill
Nicolaas E.P. Deutz, Krista L. Haines, Paul E. Wischmeier, Mariëlle P.K.J. Engelen
Current Opinion in Clinical Nutrition & Metabolic Care.2024; 27(1): 61. CrossRef
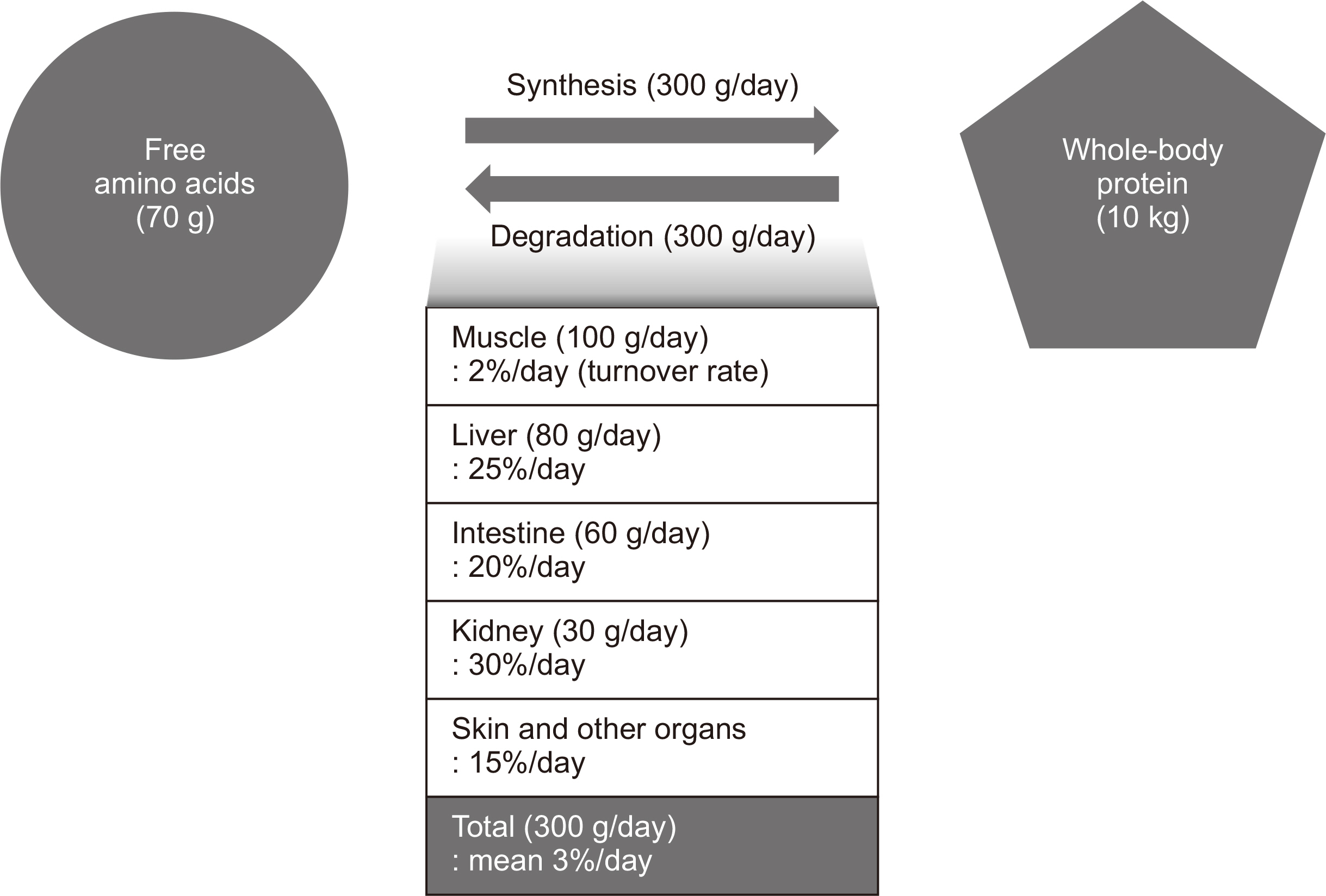
Fig. 1
Muscle protein metabolism in various clinical conditions
| MPS | MPB | NPB | |
|---|---|---|---|
| Normal (post-prandial) | ++ | + | + |
| Normal (fasting) | + | ++ | - |
| Catabolic state (fasting) | +++ | ++++++ | --- |
| Catabolic state (protein supply) | ++++ | ++++++ | -- |
| Catabolic state (rehabilitation) | ? | ? | - |
The number of (+) or (–) signs indicates the relative rate of synthesis or breakdown of muscle protein, respectively. MPS = muscle protein synthesis; MPB = muscle protein breakdown; NPB = net protein balance.
The number of (+) or (–) signs indicates the relative rate of synthesis or breakdown of muscle protein, respectively. MPS = muscle protein synthesis; MPB = muscle protein breakdown; NPB = net protein balance.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

