Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 11(2); 2020 > Article
- Original Article Efficacy of Intravenous Ferric Carboxymaltose in Patients with Acute Post-Operative Anemia after Colorectal Cancer Surgery
-
Young Ju Oh, M.D., Dae Hee Pyo, M.D., Jung Kyong Shin, M.D., Yoon Ah Park, M.D., Jung Wook Huh, M.D., Hee Cheol Kim, M.D., Seong Hyeon Yun, M.D., Woo Yong Lee, M.D., Yong Beom Cho, M.D., Ph.D.

-
Surgical Metabolism and Nutrition 2020;11(2):61-65.
DOI: https://doi.org/10.18858/smn.2020.11.2.61
Published online: December 30, 2020
Department of General Surgery, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea
- Corresponding author: Yong Beom Cho E-mail gscyb@skku.edu ORCID https://orcid.org/0000-0002-9944-4706
Copyright © 2020 The Korean Society of Surgical Metabolism and Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,832 Views
- 1 Download
- 2 Crossref
Abstract
-
Purpose This study evaluated the efficacy of administering intravenous ferric carboxymaltose (Ferinject®) in patients with acute post-operative anemia after colorectal surgery.
-
Materials and Methods Patients with colorectal cancer, who underwent colectomy from January 2017 to July 2019 at the Samsung Medical Center, were retrospectively reviewed. Depending on their body weight, patients were administered 500 mg or 1000 mg of ferric carboxymaltose (ferric carboxymaltose group, 92 patients). The primary outcome evaluated was serum hemoglobin level on post-operative day 21. The secondary outcome was the number of hemoglobin responders, defined as the proportion of patients with normalized serum hemoglobin on post-operative day 21 (hemoglobin level of 12 g/dL or more for male, 11 g/dL or more for female), and a serum hemoglobin increase of 2 g/dL or more from baseline (day 0).
-
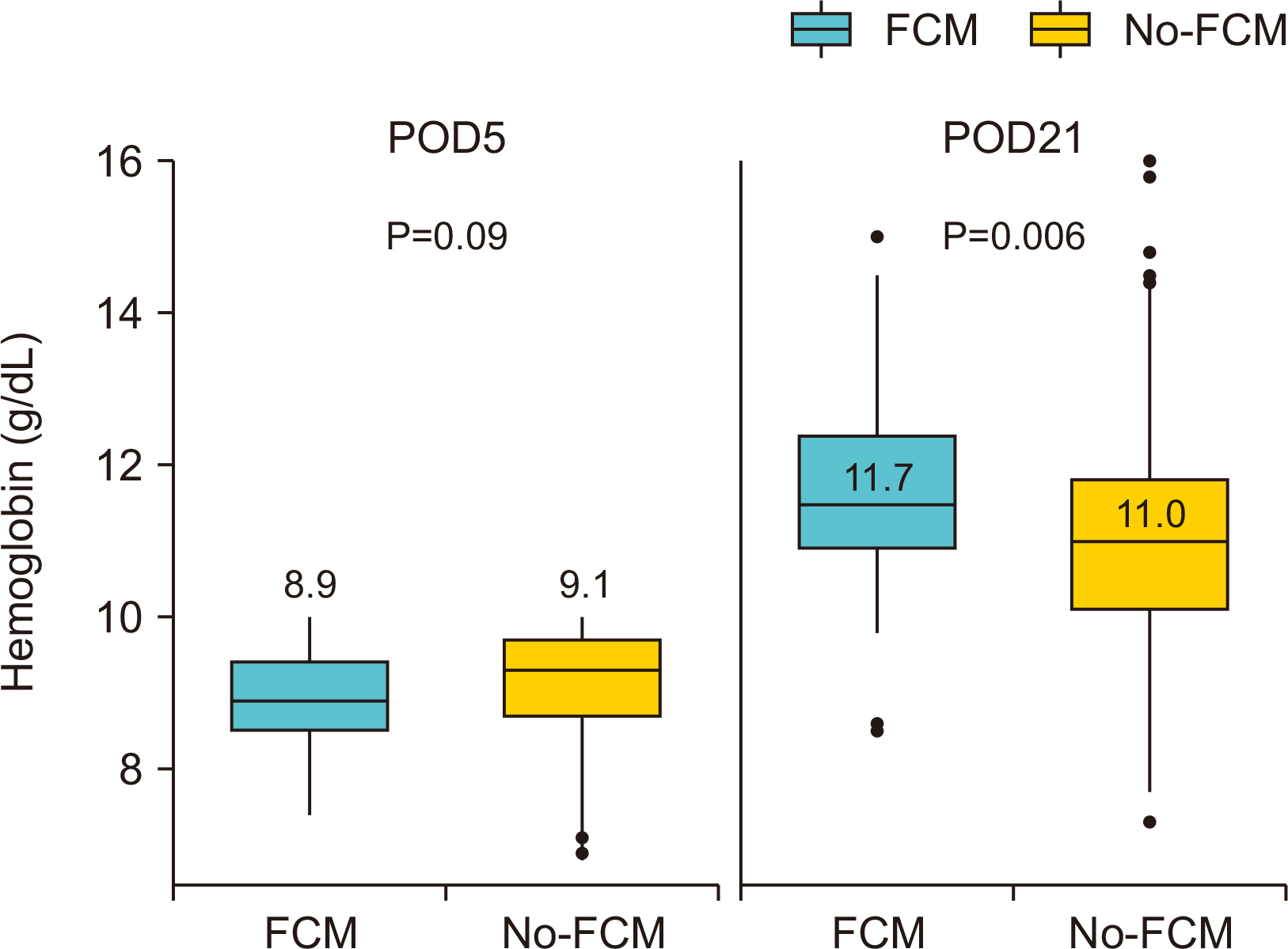
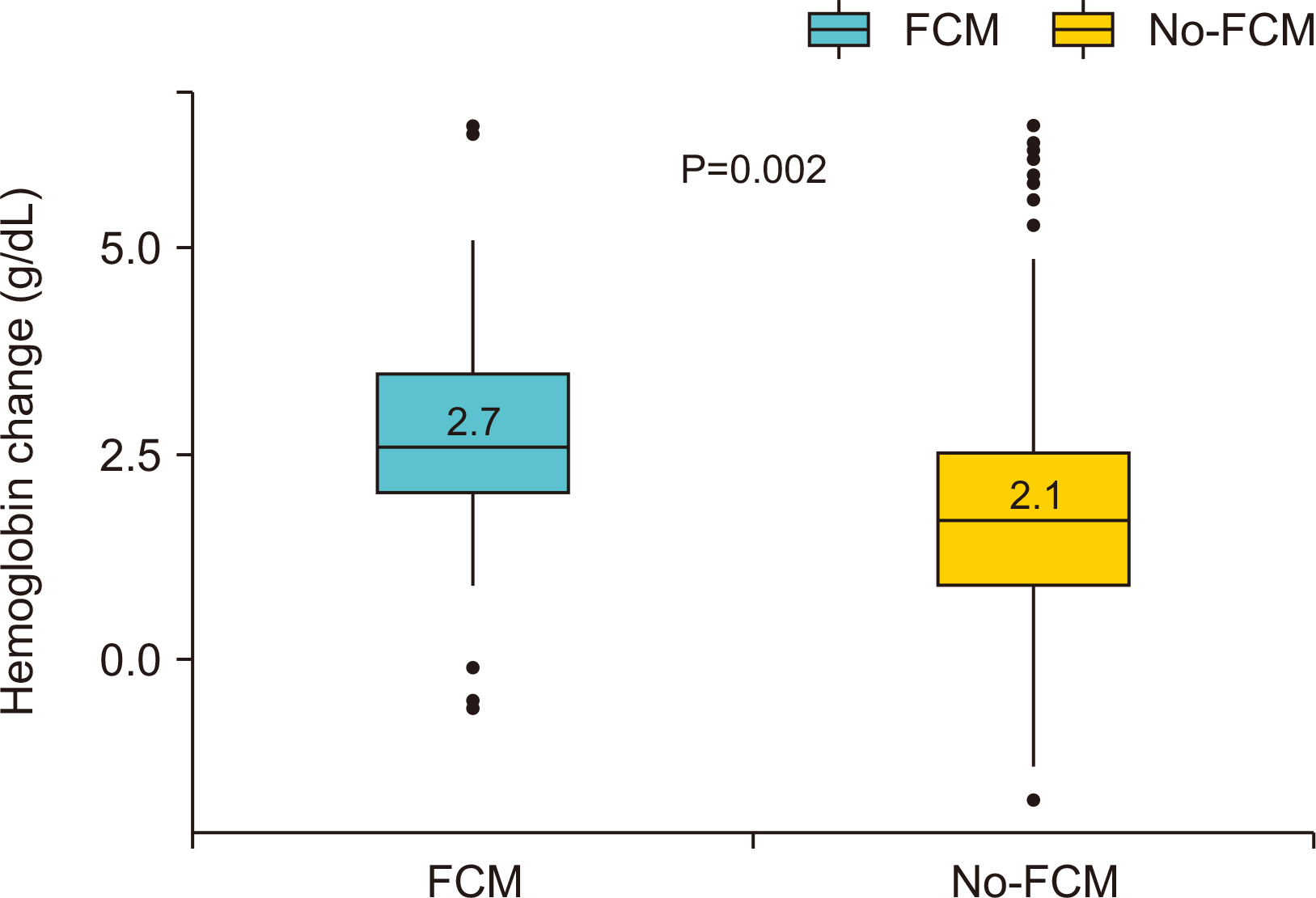
Results Of the 560 patients who underwent colectomy, 92 patients (median age, 67 years; women 59.8%; mean baseline hemoglobin level 10.7 g/dL) received intravenous ferric carboxymaltose. Compared with the no-ferric carboxymaltose group, patients in the ferric carboxymaltose group experienced significantly greater improvement in serum hemoglobin level (post-operative day 5: 8.9 g/dL, P=0.09; post-operative day 21: 11.7 g/dL, P=0.006). The increase in hemoglobin levels was significantly greater in the ferric carboxymaltose group (2.7 g/dL) than the no-ferric carboxymaltose group (2.1 g/dL) (P=0.002).
-
Conclusion Findings of this study indicate a better outcome after administering intravenous ferric carboxymaltose, which results in continuous increase in the levels of hemoglobin during the early post-operative period after colorectal cancer surgery.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. ArticlePubMedPDF
- 2. Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep 2018;8:13345.ArticlePubMedPMCPDF
- 3. Calleja JL, Delgado S, del Val A, Hervás A, Larraona JL, Terán Á, et al. Colon Cancer Study Group. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int J Colorectal Dis 2016;31:543-51. ArticlePubMedPDF
- 4. Kim YW, Bae JM, Park YK, Yang HK, Yu W, Yook JH, et al. FAIRY Study Group. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. JAMA 2017;317:2097-104. ArticlePubMedPMC
- 5. Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i. Ann Oncol 2012;23:1954-62. ArticlePubMed
- 6. Ansarinejad N, Abbasi B, Sadat Rasul MS, Fardad F, Ramim T. The effectiveness of ferric carboxymaltose on the improvement of chronic iron deficiency anemia in patients with colon cancer: a controlled randomized clinical trial. Obstet Gynecol Cancer Res 2016;1:e8006. ArticlePDF
- 7. Borstlap WAA, Buskens CJ, Tytgat KMAJ, Tuynman JB, Consten ECJ, Tolboom RC, et al. 2015;Multicentre randomized controlled trial comparing ferric(III)carboxymaltose infusion with oral iron supplementation in the treatment of preoperative anaemia in colorectal cancer patients. BMC Surg 15:78.ArticlePubMedPMCPDF
- 8. Muñoz M, Gómez-Ramírez S, Martín-Montañez E, Auerbach M. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol 2014;20:1972-85. ArticlePubMedPMC
- 9. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214-21. ArticlePubMed
- 10. Tartter PI, Quintero S, Barron DM. Perioperative blood transfusion associated with infectious complications after colorectal cancer operations. Am J Surg 1986;152:479-82. ArticlePubMed
- 11. Tartter PI. Blood transfusion and infectious complications following colorectal cancer surgery. Br J Surg 1988;75:789-92. ArticlePubMedPDF
- 12. Laso-Morales MJ, Vives R, Vallejo-Tarrat A, Caló N, Valle-Beltran A, Pontes C. Single dose of intravenous ferric carboxymaltose infusion versus multiple fractionated doses of intravenous iron sucrose in the treatment of postoperative anaemia in colorectal cancer patients: study protocol for a randomised controlled trial. Trials 2019;20:23.ArticlePubMedPMCPDF
- 13. Schreiber S, Howaldt S, Schnoor M, Nikolaus S, Bauditz J, Gasché C, et al. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med 1996;334:619-23. ArticlePubMed
- 14. Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther 2006;24:1507-23. ArticlePubMed
- 15. Muñoz M, Gómez-Ramírez S, Cuenca J, García-Erce JA, Iglesias-Aparicio D, Haman-Alcober S, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 2014;54:289-99. ArticlePubMed
- 16. Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth 2011;107:477-8. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

- Prevalence and factors influencing anemia recovery after intensive care
Kyoung Won Yoon, Sungjoo Park, Chi-Min Park
Transfusion and Apheresis Science.2024; 63(3): 103922. CrossRef - Effect of intraoperative intravenous ferric derisomaltose supplementation on reduction of postoperative anemia and transfusion in chronic kidney disease patients after total knee replacement
Jae Hyun Kwon, Yong Hyun Cho, Won Jang, Sun Hee Kim, Hyun Cheol Ko, Woo Hyeong Ko, Young Do Kim
Medicine.2022; 101(35): e30105. CrossRef
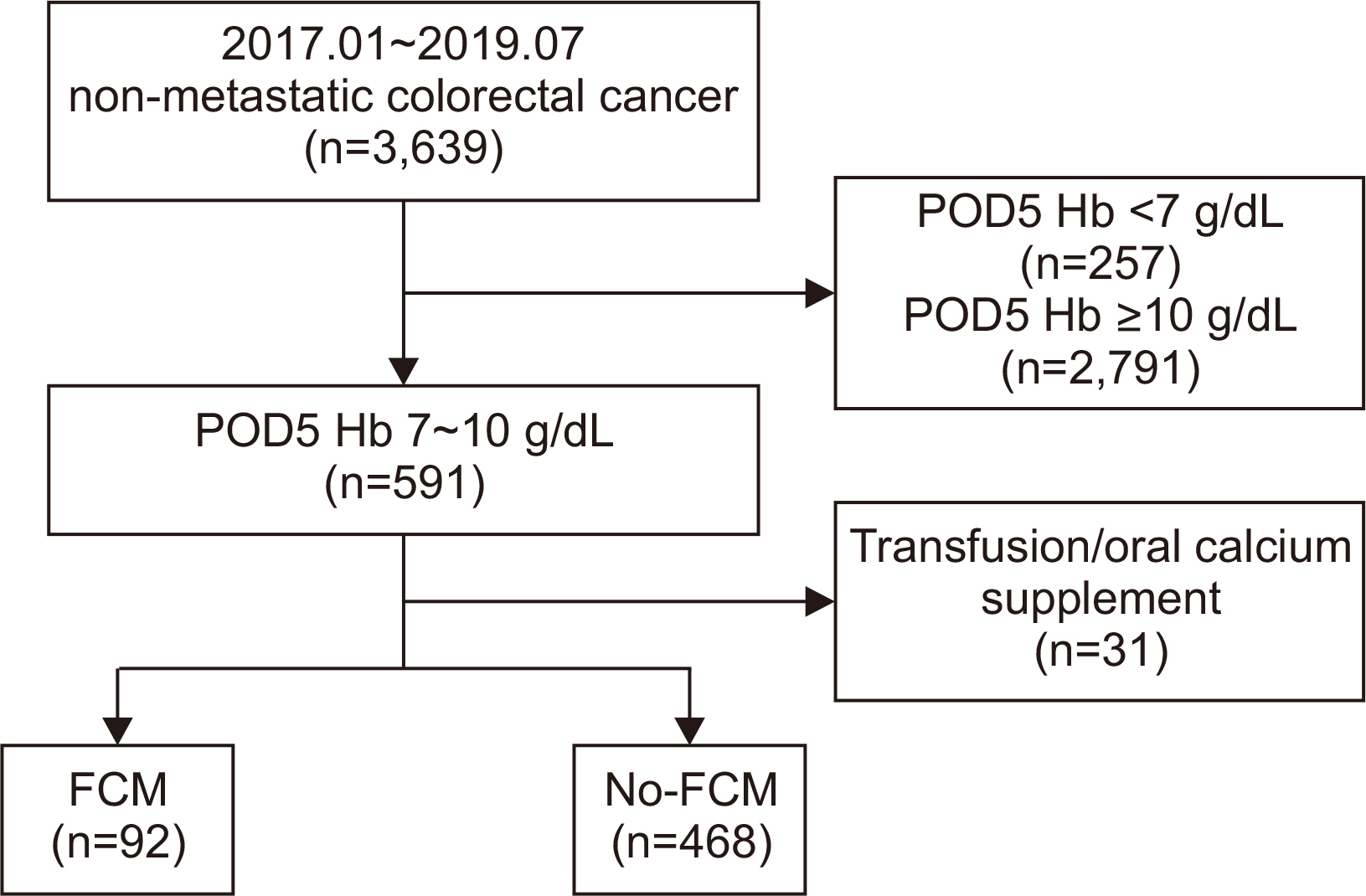
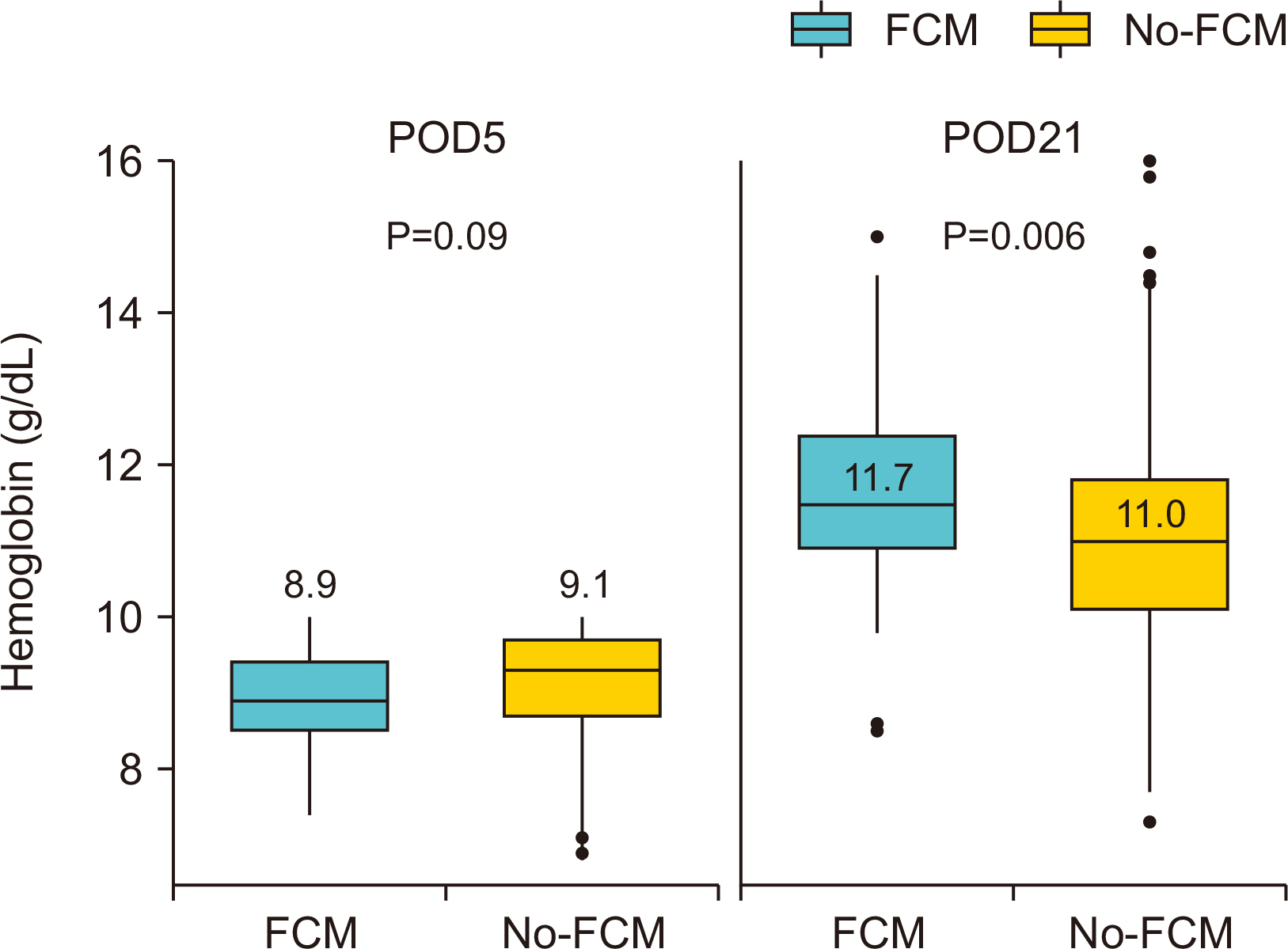
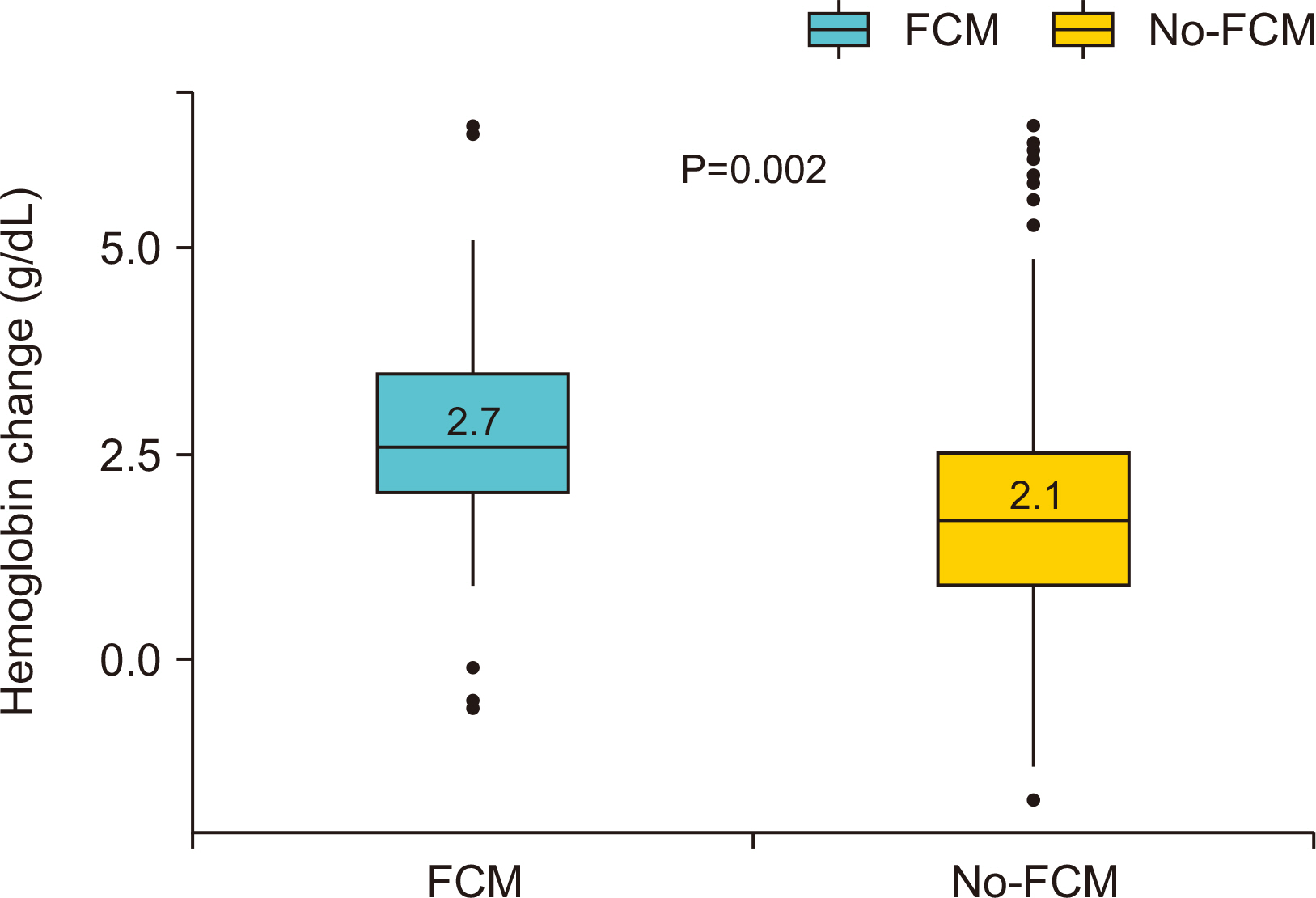
Fig. 1
Fig. 2
Fig. 3
Baseline characteristics for patients with acute postoperative anemia following colectomy receiving ferric carboxymaltose (FCM) vs. no-ferric carboxymaltose (no-FCM)
| FCM (n=92) | no-FCM (n=468) | P | |
|---|---|---|---|
| Sex, n (%) | 0.657 | ||
| Female | 55 (59.8) | 265 (56.6) | |
| Male | 30 (40.2) | 203 (43.4) | |
| Age, median (IQD) | 67 (56~77) | 63 (54~74) | 0.045 |
| BMI, mean (SD), kg/m2 | 23.5 (3.6) | 23.3 (3.6) | 0.576 |
| Preoperative Hct, mean (SD), % | 34.0 (4.1) | 34.7 (4.4) | 0.174 |
| Preoperative Hb, mean (SD), g/dL | 10.7 (1.6) | 11.0 (1.7) | 0.159 |
| Tumor location, n (%) | 0.075 | ||
| Colon | 63 (68.5) | 364 (77.8) | |
| Rectum | 29 (31.5) | 104 (22.2) | |
| Type of operation, n (%) | 0.463 | ||
| MIS (Lap, SILS, Robot) | 74 (80.4) | 394 (84.2) | |
| Open | 18 (19.6) | 74 (15.8) | |
| Name of operation, n (%) | 0.120 | ||
| Colectomy | 51 (33.4) | 231 (49.4) | |
| AR/LAR | 38 (41.3) | 232 (49.6) | |
| APR | 3 (3.3) | 5 (1.1) | |
| EBL, n (%) | 0.101 | ||
| <100 mL | 47 (51.1) | 263 (56.2) | |
| 100 mL~500 mL | 45 (48.9) | 190 (40.6) | |
| ≥500 mL | 0 (0) | 15 (3.2) | |
| AJCC 7th staging, n (%) | 0.001 | ||
| I | 34 (37.0) | 92 (19.7) | |
| II | 29 (31.5) | 193 (41.2) | |
| III | 29 (31.5) | 183 (39.1) |
Hct = hematocrit; Hb = hemoglobin; MIS = minimally invasive surgery; Lap = laparoscopic surgery; SILS = single incision laparoscopic; AR = anterior resection; LAR = low anterior resection; APR = abdominoperineal resection; EBL = estimate blood loss.
Comparison of outcomes between receiving ferric carboxymaltose (FCM) vs. no-ferric carboxymaltose (no-FCM)
| FCM (n=92) | no-FCM (n=468) | P | |
|---|---|---|---|
| △Hb (POD21?POD5), mean (SD), g/dL | 2.7 (1.3) | 2.1 (1.3) | 0.002 |
| Responder, n (%) | <0.0001 | ||
| (+) | 77 (83.7) | 257 (54.9) | |
| (-) | 15 (16.3) | 211 (45.1) | |
| Hb increase from baseline, n (%) | <0.0001 | ||
| ≥ 2 g/dL | 71 (77.2) | 193 (41.2) | |
| <2 g/dL | 21 (22.8) | 275 (58.8) | |
| Normalized at POD 21, n (%) | 68 (73.9) | 237 (50.6) | <0.0001 |
Hb = hemoglobin; POD = Post-op day.
Hct = hematocrit; Hb = hemoglobin; MIS = minimally invasive surgery; Lap = laparoscopic surgery; SILS = single incision laparoscopic; AR = anterior resection; LAR = low anterior resection; APR = abdominoperineal resection; EBL = estimate blood loss.
Hb = hemoglobin; POD = Post-op day.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN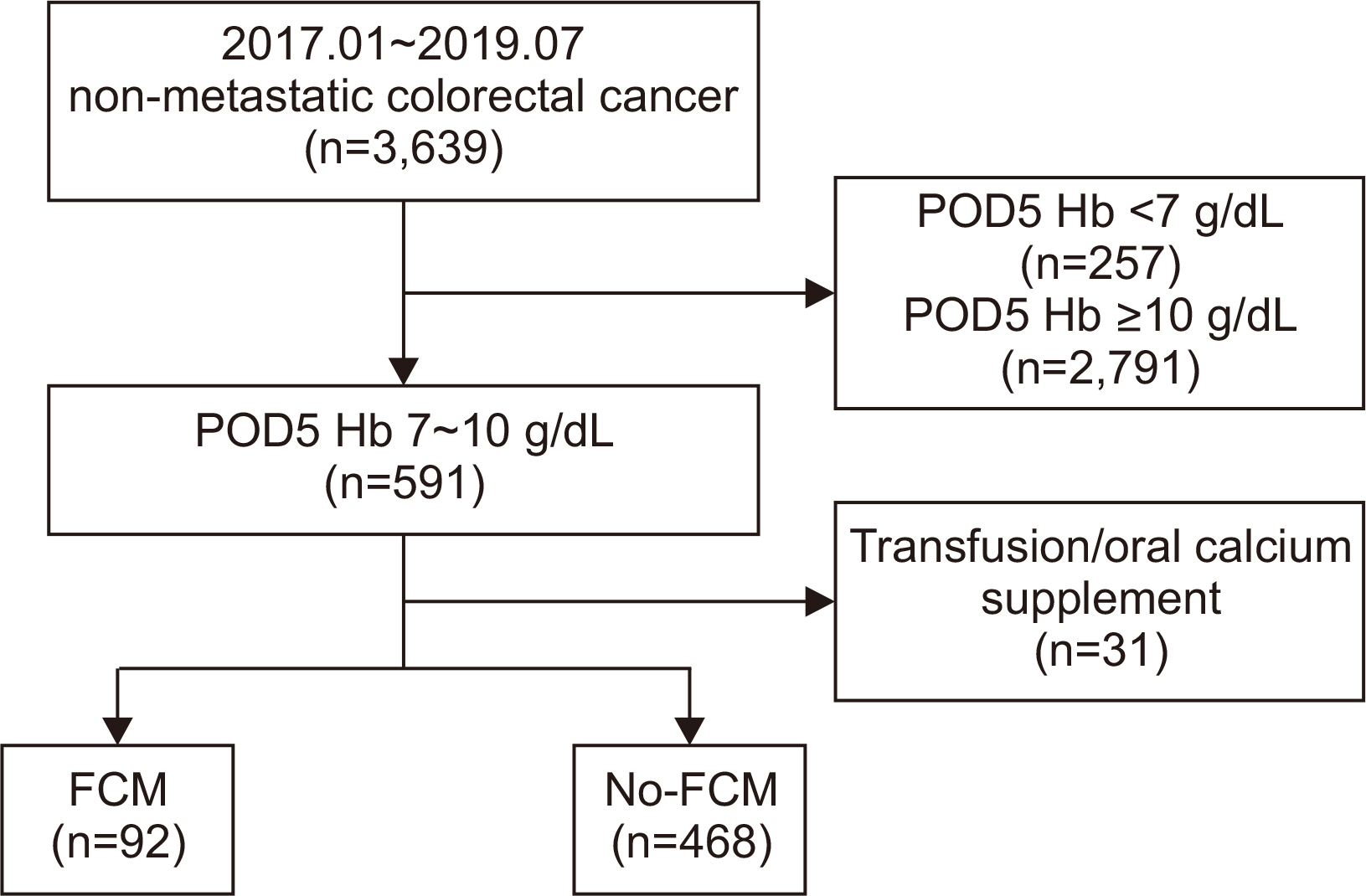
 Cite
Cite

