Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 15(1); 2023 > Article
- Review Efficacy of monounsaturated fatty acids in reducing risk of the cardiovascular diseases, cancer, inflammation, and insulin resistance: a narrative review
-
Ki Hyun Kim
 , Yoonhong Kim
, Yoonhong Kim , Kyung Won Seo
, Kyung Won Seo
-
Annals of Clinical Nutrition and Metabolism 2023;15(1):2-7.
DOI: https://doi.org/10.15747/ACNM.2023.15.1.2
Published online: April 1, 2023
Department of Surgery, Kosin University College of Medicine, Busan, Korea
- Corresponding author: Kyung Won Seo, email: kwseo@office.kosin.ac.kr
© 2023 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 33,193 Views
- 96 Download
- 10 Crossref
- Abstract
- Introduction
- Potential health benefits of olive oil
- Benefit of lipids as a source of energy and their relationship with immunity
- History of intravenous lipid emulsions
- Characteristics and effects of olive oil-based LEs
- The benefits of MUFAs focusing on the guidelines
- Conclusion
- Acknowledgments
- Notes
- References
Abstract
-
Purpose The purpose of this review is to explore the potential benefits of monounsaturated fatty acids (MUFAs), specifically those found in olive oil, on weight loss, cardiovascular disease, cancer, inflammation, and insulin resistance. Additionally, this review examines the use of olive oil–based intravenous lipid emulsions (ILEs) in providing parenteral nutrition to patients with diverse needs.
-
Current concept MUFAs, found in olive oil, nuts, and some animal foods, have been found to have numerous health benefits. A diet high in MUFAs can aid in weight loss and reduce the risk of cardiovascular disease. Olive oil, in particular, has been linked to a lower risk of cancer, inflammation, and insulin resistance. In addition, olive oil–based ILEs have been utilized for over two decades and are well tolerated by patients requiring parenteral nutrition.
-
Conclusion A diet rich in MUFAs, specifically from olive oil, can provide numerous health benefits, including weight loss and reducing the risk of cardiovascular disease, cancer, inflammation, and insulin resistance. Additionally, olive oil–based ILEs have been shown to effectively provide nutrients to diverse populations requiring parenteral nutrition and have demonstrated the ability to preserve immune function and induce less lipid peroxidation than other ILEs. Further research is needed to fully understand the potential benefits of MUFAs and olive oil-based ILEs, but current evidence suggests that they may be a valuable addition to a healthy diet and medical treatment.
Introduction
Potential health benefits of olive oil
Benefit of lipids as a source of energy and their relationship with immunity
History of intravenous lipid emulsions
Characteristics and effects of olive oil-based LEs
The benefits of MUFAs focusing on the guidelines
Conclusion
Acknowledgments
Authors’ contribution
Conceptualization: KWS. Formal analysis: KHK, YK. Methodology: KHK. Project administration: KHK, KWS. Supervision: KWS. Validation: KHK, KWS. Visualization: KHK, YK. Writing – original draft: KHK. Writing – review & editing: KHK, KWS.
Conflict of interest
Kyung Won Seo is an editorial board member of the journal, but was not involved in the review process of this manuscript. Otherwise, there is no conflict of interest to declare.
Funding
None.
Data availability
None.
Supplementary materials
None.
Revised from the article of Mirtallo et al. (Nutr Clin Pract 2020;35:769-82) [7] with original copyright holder’s permission.
ILE = lipid injectable emulsion; MCT = medium-chain triglyceride; EFA = essential fatty acid; FA = fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; IFALD = intestinal failure-associated liver disease.
- 1. Gomes F, Schuetz P, Bounoure L, Austin P, Ballesteros-Pomar M, Cederholm T, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr 2018;37:336-53. ArticlePubMed
- 2. Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 2007;85:1171-84. ArticlePubMed
- 3. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids and risk of cardiovascular disease: synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012;4:1989-2007. ArticlePubMedPMC
- 4. Cao X, Xia J, Zhou Y, Wang Y, Xia H, Wang S, et al. The effect of MUFA-rich food on lipid profile: a meta-analysis of randomized and controlled-feeding trials. Foods 2022;11:1982.ArticlePubMedPMC
- 5. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911-9. ArticlePubMed
- 6. Qian F, Korat AA, Malik V, Hu FB. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2016;39:1448-57. ArticlePubMedPMCPDF
- 7. Mirtallo JM, Ayers P, Boullata J, Gura KM, Plogsted S, Anderson CR, et al. ASPEN lipid injectable emulsion safety recommendations, part 1: background and adult considerations. Nutr Clin Pract 2020;35:769-82; Erratum in: Nutr Clin Pract 2022;37:482. ArticlePubMedPDF
- 8. Calder PC, Adolph M, Deutz NE, Grau T, Innes JK, Klek S, et al. Lipids in the intensive care unit: recommendations from the ESPEN Expert Group. Clin Nutr 2018;37:1-18. ArticlePubMed
- 9. Wu GH, Zaniolo O, Schuster H, Schlotzer E, Pradelli L. Structured triglycerides versus physical mixtures of medium- and long-chain triglycerides for parenteral nutrition in surgical or critically ill adult patients: systematic review and meta-analysis. Clin Nutr 2017;36:150-61. ArticlePubMed
- 10. Demirer S, Sapmaz A, Karaca AS, Kepenekci I, Aydintug S, Balci D, et al. Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann Surg Treat Res 2016;91:309-15. ArticlePubMedPMCPDF
- 11. Reimund JM, Scheer O, Muller CD, Pinna G, Duclos B, Baumann R. In vitro modulation of inflammatory cytokine production by three lipid emulsions with different fatty acid compositions. Clin Nutr 2004;23:1324-32. ArticlePubMed
- 12. Pontes-Arruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med 2006;34:2325-33. ArticlePubMed
- 13. Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr 2006;30:351-67. ArticlePubMedPDF
- 14. Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJ. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med 2010;36:735-49. ArticlePubMedPMC
- 15. Jia ZY, Yang J, Xia Y, Tong DN, Zaloga GP, Qin HL. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: a prospective, randomized, multi-center clinical trial in China. Nutr J 2015;14:119.ArticlePubMedPMC
- 16. Huschak G, Zur Nieden K, Hoell T, Riemann D, Mast H, Stuttmann R. Olive oil based nutrition in multiple trauma patients: a pilot study. Intensive Care Med 2005;31:1202-8. ArticlePubMedPDF
- 17. Umpierrez GE, Spiegelman R, Zhao V, Smiley DD, Pinzon I, Griffith DP, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 2012;40:1792-8. ArticlePubMedPMC
- 18. Mateu-de Antonio J, Grau S, Luque S, Marín-Casino M, Albert I, Ribes E. Comparative effects of olive oil-based and soyabean oil-based emulsions on infection rate and leucocyte count in critically ill patients receiving parenteral nutrition. Br J Nutr 2008;99:846-54. ArticlePubMed
- 19. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. Guidelines on parenteral nutrition: surgery. Clin Nutr 2009;28:378-86. ArticlePubMed
- 20. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623-50. ArticlePubMed
- 21. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. ArticlePubMed
- 22. Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, et al. A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract 2012;27:150-92. ArticlePubMed
- 23. Anez-Bustillos L, Dao DT, Baker MA, Fell GL, Puder M, Gura KM. Intravenous fat emulsion formulations for the adult and pediatric patient: understanding the differences. Nutr Clin Pract 2016;31:596-609. ArticlePubMedPMC
References
Figure & Data
REFERENCES
Citations

- Effects of daily extra virgin olive oil consumption on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis
Jéssica Vidal Damasceno, Anderson Garcez, Andressa Anelo Alves, Isabella Rosa da Mata, Simone Morelo Dal Bosco, Juliano Garavaglia
Critical Reviews in Food Science and Nutrition.2026; 66(2): 392. CrossRef - Applying Wild Mistol Fruits (Sarcomphalus Mistol) from the Paraguayan Chaco as Value-Added Food Ingredients
Villalba R., Belotto J., Coronel E., Carvajal M., Recalde C., Caballero S., Friesen A., Mereles L.
Plant Foods for Human Nutrition.2026;[Epub] CrossRef - Multi-Omics Integration Reveals Key Genes, Metabolites and Pathways Underlying Meat Quality and Intramuscular Fat Deposition Differences Between Tibetan Pigs and Duroc × Tibetan Crossbred Pigs
Junda Wu, Qiuyan Huang, Baohong Li, Zixiao Qu, Xinming Li, Fei Li, Haiyun Xin, Jie Wu, Chuanhuo Hu, Sen Lin, Xiangxing Zhu, Dongsheng Tang, Chuang Meng, Zongliang Du, Erwei Zuo, Fanming Meng, Sutian Wang
Animals.2026; 16(2): 214. CrossRef - The MetaboHealth Score Enhances Insulin Resistance Metabotyping for Targeted Fat Loss: The PERSON Study
Jordi Morwani‐Mangnani, Fatih A. Bogaards, Alexander Umanets, Gabby B. Hul, Anouk Gijbels, Gijs H. Goossens, Joris Deelen, Marian Beekman, Lydia Afman, Ellen E. Blaak, P. Eline Slagboom
Obesity.2026;[Epub] CrossRef - Video and Text‐Based Supplemental Health Information and Consumer Willingness to Pay for Nutrient‐Enhanced Eggs
Edeoba W. Edobor, Michael J. Best, Anita R. Best, Ondulla T. Toomer
Agribusiness.2026;[Epub] CrossRef - The impact of Lactiplantibacillus plantarum on the cream composition: Insight into changes of vitamin D3 content and fatty acid composition
Tetiana Dyrda-Terniuk, Viorica Railean, Aleksandra Bogumiła Florkiewicz, Justyna Walczak-Skierska, Mateusz Kolankowski, Joanna Rudnicka, Dorota Białczak, Paweł Pomastowski
International Dairy Journal.2025; 161: 106118. CrossRef - Palmitoleic and oleic fatty acids as biomarkers for coronary heart disease: A predictive model
Guangzhou Wang, Lin Zhou, Zhengfang Wang, Asmaa Ali, Liang Wu
Irish Journal of Medical Science (1971 -).2025; 194(1): 59. CrossRef - Macrophages: their role in immunity and their relationship with fatty acids in health and disease
Mayte Rueda-Munguía, Luis Alberto Luévano-Martínez, Gerardo García-Rivas, Elena Cristina Castillo, Omar Lozano
Frontiers in Immunology.2025;[Epub] CrossRef - Evaluation of the Nutritional Value of Prunus dulcis Blossoms and the Antioxidant Compounds of Their Extracted Oil Using Green Extraction Method
Theodoros Chatzimitakos, Vassilis Athanasiadis, Konstantina Kotsou, Ioannis Makrygiannis, Eleni Bozinou, Stavros I. Lalas
Applied Sciences.2024; 14(5): 2001. CrossRef - Oleic Acid and Succinic Acid: A Potent Nutritional Supplement in Improving Hepatic Glycaemic Control in Type 2 Diabetic Sprague–Dawley Rats
Kemmoy G. Lattibeaudiere, Ruby Lisa Alexander-Lindo, Mozaniel Oliveira
Advances in Pharmacological and Pharmaceutical Sciences.2024;[Epub] CrossRef
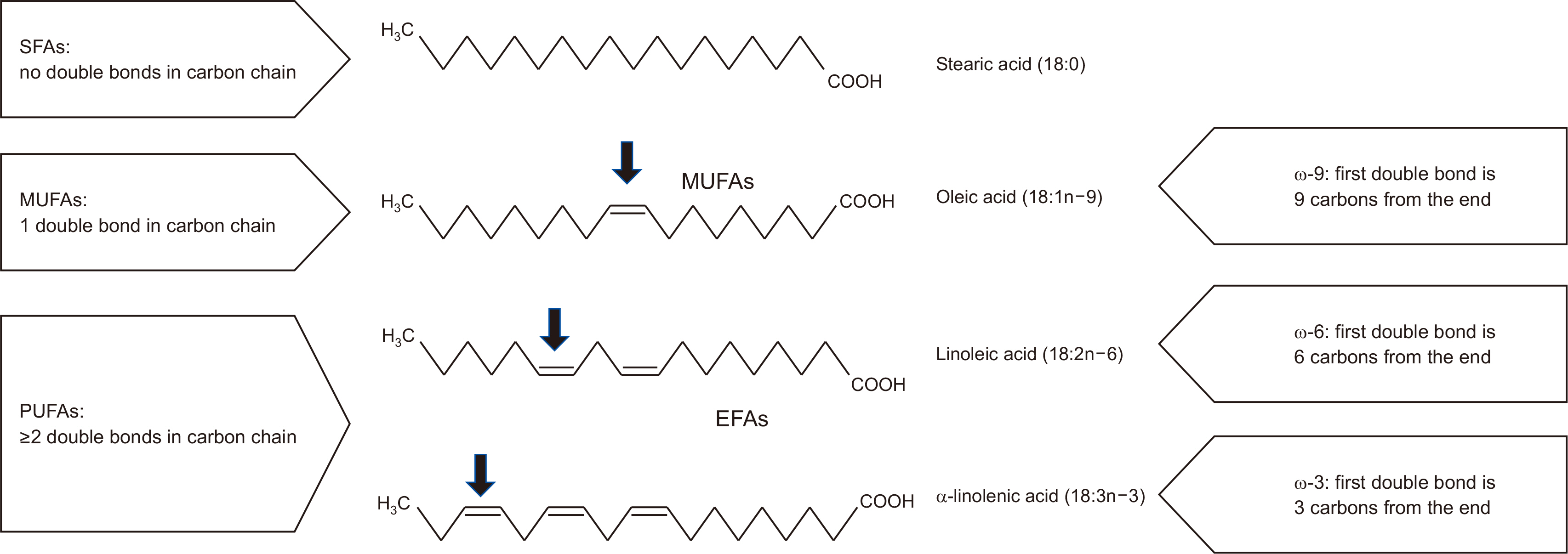
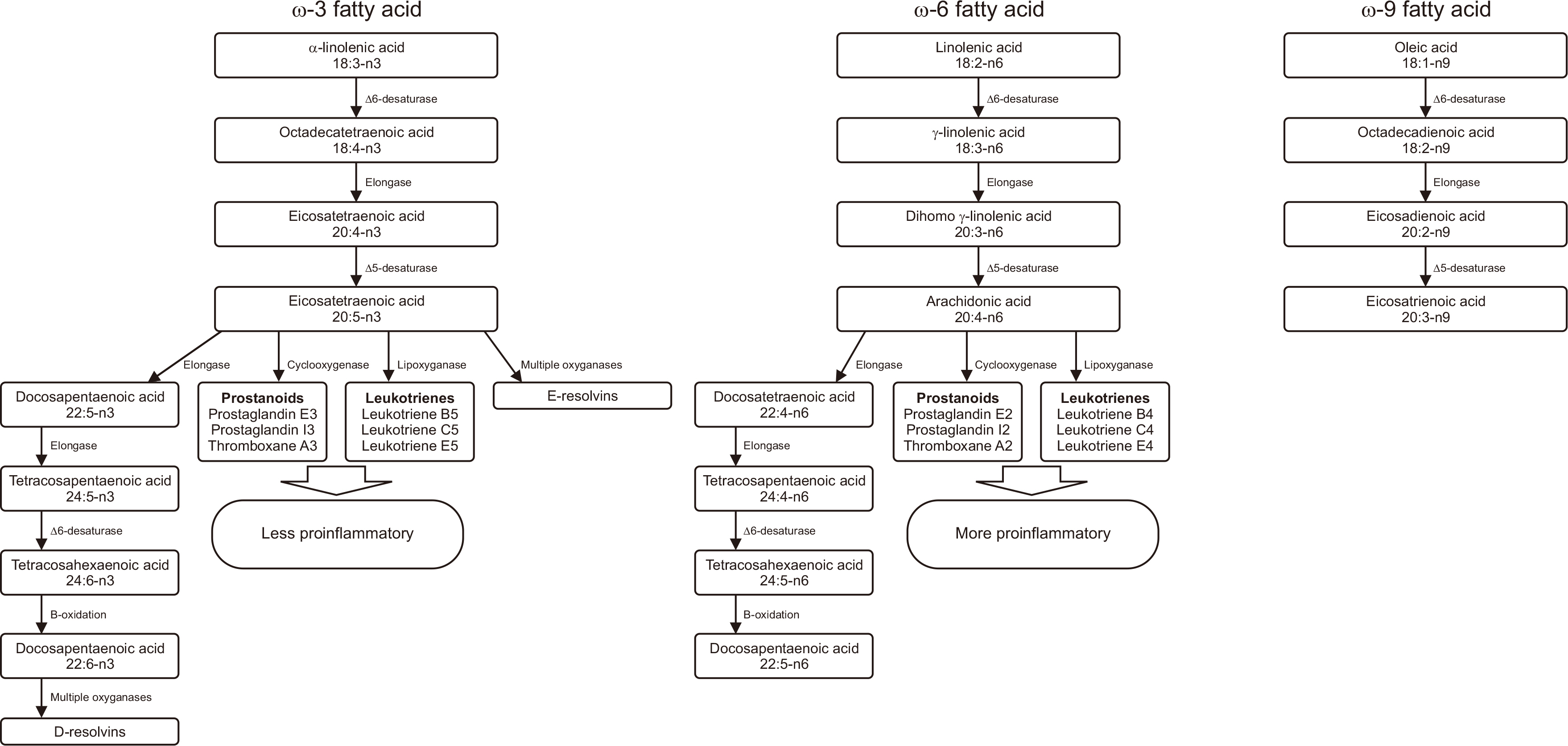
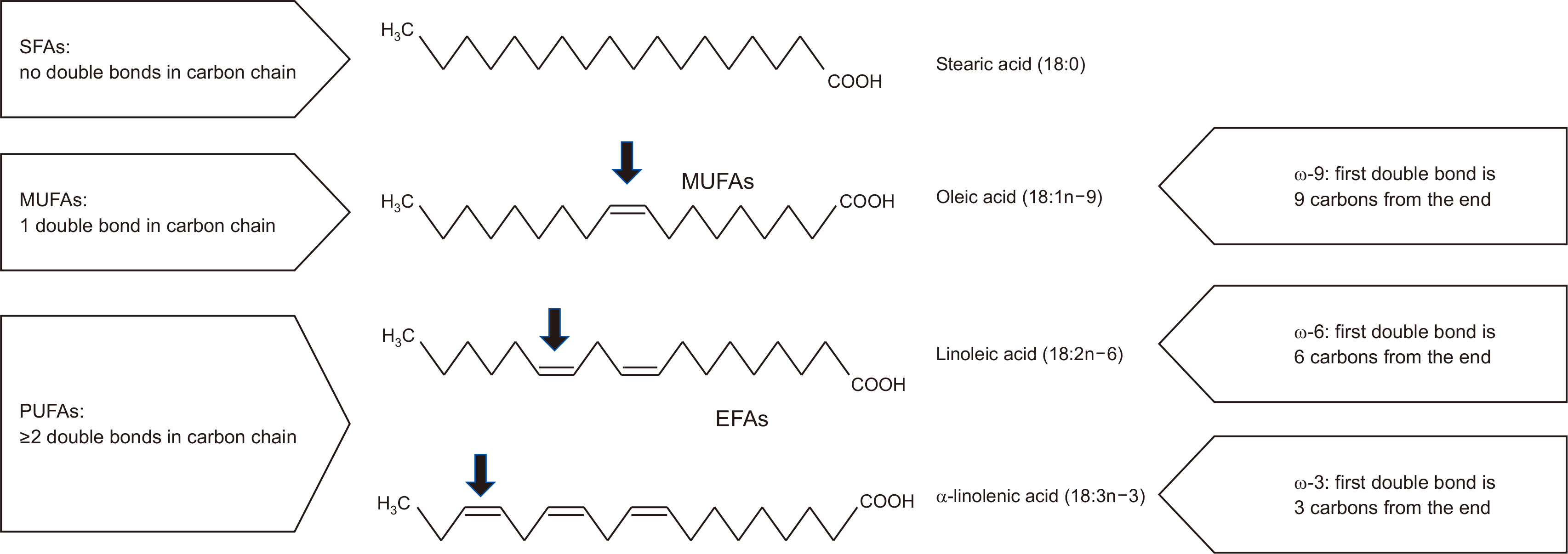
Fig. 1
Fig. 2
Evolution of intravenous lipid emulsions
| Generation of ILE | Description | Potential benefits |
|---|---|---|
| First | Soybean oil or safflower oil | Information about compatibility with regularly used drugs is provided |
| Second | Two-oil formulation including soybean oil and MCT | MCTs are removed more quickly and with less peroxidation |
| Third | Two-oil ILE using soybean oil and olive oil, resulting in a reduced amount of EFA (ω-6 FAs) | Elevated doses of MUFAs produce fewer peroxides during oxidation than PUFAs Oleic acid in olive oil is not converted to inflammatory or immune mediators Patients who are at risk of immunosuppression or have impaired immune systems may benefit from this treatment |
| Fourth | Four-oil ILE of soybean oil, MCT, olive oil, and fish oil | Fish oil included for critically ill and surgical patient populations |
| Fish oil | Sources of FA and energy for infants and children with IFALD and may reverse IFALD |
Revised from the article of Mirtallo et al. (Nutr Clin Pract 2020;35:769-82) [
ILE = lipid injectable emulsion; MCT = medium-chain triglyceride; EFA = essential fatty acid; FA = fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; IFALD = intestinal failure-associated liver disease.
Revised from the article of Mirtallo et al. (Nutr Clin Pract 2020;35:769-82) [ ILE = lipid injectable emulsion; MCT = medium-chain triglyceride; EFA = essential fatty acid; FA = fatty acid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; IFALD = intestinal failure-associated liver disease.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

