Abstract
-
Purpose
This study investigated the effects of preoperative nutritional status on postoperative outcomes in older adult patients with pancreatic adenocarcinoma.
-
Methods
The background and perioperative factors of patients who underwent pancreatectomy for pancreatic adenocarcinoma between 2007 and 2020 were retrospectively analyzed.
-
Results
Patients aged 75 years or over (older adults) were significantly associated with hypertension, upfront surgery, and lower prognostic nutritional index. In addition, these patients had a significantly lower rate of portal vein resection, less blood loss, and shorter operation time than patients aged less than 75 years (non-older adults). During the postoperative course, older adult patients had a higher rate of pneumonia and lower overall survival than younger patients, although recurrence‐free survival was comparable. In addition, older adult patients showed preoperative malnutrition as a risk factor for postoperative in‐hospital death.
-
Conclusion
Surgical treatment for pancreatic cancer in older adult patients was performed safely. However, preoperative malnutrition is a risk factor for in‐hospital death and such patients require nutritional support and less‐invasive surgery.
-
Keywords: Aged; Nutritional status; Pancreatectomy; Pancreatic neoplasm; Prognosis
Introduction
Background
Japan has entered a full-fledged aging society with a declining birthrate. The late-stage older adult population accounted for 17.48 million, or 13.8% of the total population, in fiscal year 2009 [
1]. The incidence of pancreatic cancer and biliary tract cancer has been increasing in recent years, and it is not uncommon to perform difficult hepatobiliary and pancreatic surgery on older adult patients. Although the application of highly invasive hepatobiliary and pancreatic surgery requires sufficient verification, there is no clear indicator to determine the indication for surgery in older adult patients, which is currently left to the attending physician or each institution.
The CONUT value [
2] and Onodera's prognostic nutritional index (PNI) [
3] are nutritional indices that can be calculated from daily blood sampling data, are easy to use, and can be performed at general facilities. We selected these two indicators and investigated their usefulness as perioperative risk assessment factors for the nutritional status of older adult (≥75 years) and non-older adult (<75 years) patients with pancreatic cancer.
Methods
Ethics statement
This study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine (2020-1-322) as a “Study of clinicopathologically relevant factors and treatment outcomes in pancreatic diseases." The written informed consent was waived because this design is a retrospective study.
Study design
Setting
This study was done at Tohoku University Hospital between January 2007 and June 2020. Surgical procedure for pancreatic cancer patients were as follows:
The standard pancreaticoduodenectomy (PD) for pancreatic cancer in our department is a subtotal stomach-sparing PD in which the stomach is orally dissected 2-4 cm from the pyloric ring and standard lymph node dissection. The modified Child method is used for reconstruction, and the Blumgart method is mainly used for pancreaticojejunostomy since 2016, although the two-layer pancreaticojejunostomy was used until 2015 [
4]. An enteral feeding tube is also implanted and postoperative enteral nutrition is used. Distal pancreatectomy (DP) with standard lymph node dissection, dividing the pancreas just above the portal vein, is the basic approach for pancreatic cancer. In open DP, the main pancreatic duct is double ligated and the pancreatic stump is closed in a fish-mouth fashion; however, in laparoscopic surgery, the stump is closed with an automatic suturing device.
We included 549 patients with pancreatic cancer who underwent resection in the Department of General Surgery, Tohoku University Hospital between January 2007 and June 2020. A total of 122 patients (22.2%) were aged 75 years or older (older adult group) with the remaining 427 patients (77.8%) aged less than 75 years (non-older adult group).
Variables
The following background factors, intraoperative factors, and postoperative outcomes of the older adult and non-older adult groups were reviewed in the medical records and our department database for retrospective evaluation. Background factors included age, sex, comorbidities (hypertension, diabetes), preoperative chemotherapy, stage of disease, and preoperative nutritional indices such as PNI and CONUT values. Intraoperative factors included surgical technique, portal vein resection, operative time, blood loss, and R0 resection. The postoperative outcome measures included all complications, major complications, hospital mortality, postoperative pancreatic fistula, surgical site infection (SSI), organ space SSI, pneumonia, sepsis, thrombosis, postoperative hospital stay, readmission within 30 days, overall survival, and recurrence-free survival. Severe complications were defined as IIIa or higher of the Clavien-Dindo classification [
5], and postoperative pancreatic fistula was defined according to the criteria of the revised International Study Group on Pancreatic Surgery [
6]. Staging was described in accordance with the 7th edition of the General Rules for the Study of Pancreatic Cancer [
7]. Patients were followed up every 3 months after surgery as outpatients, and the presence or absence of recurrence was confirmed mainly by imaging tests. Overall survival and recurrence-free survival were defined as the period from the date of surgery to the date of death, the date of recurrence confirmation, or the date of the last outpatient visit, with the date of recurrence confirmation being the date when recurrence was confirmed by imaging tests. Recurrence-free survival was terminated in the case of death without recurrence.
There was no selection bias reportable.
Data sources
Data were from the patient’s medical records.
Measurements
We compared the background, intraoperative, and postoperative factors between the older adult and non-older adult patients, and confirmed the background and perioperative characteristics of older adult patients undergoing pancreatic cancer resection. The PNI and CONUT values were calculated using the following formula, and patients with PNI less than 40 were classified as malnourished.
PNI=10×(albumin value)+0.005×(total lymphocyte count), CONUT values [
2] were calculated as shown in
Fig. 1.
A score of 0 to 1 is normal, 2 to 4 is mildly abnormal, 5 to 8 is moderately abnormal, and 9 or more is severely abnormal. In this study, patients with moderate or severe abnormalities with a CONUT value of 5 or higher were defined as malnourished.
Study size
Since all target patients were recruited and included according to the selection criteria, no sample size estimation was done.
Statistical methods
Continuous variables are presented as the mean±standard deviation if they followed a normal distribution, or as the median and range if they did not. For nominal variables, either the chi-square test or the Fisher direct probability calculation method was used. Survival rates were statistically analyzed using the log-rank test with the Kaplan-Meier method. A P-value of less than 0.05 was defined as statistically significant.
Results
Characteristics of resected pancreatic cancer cases in older adult patients
A comparison of background factors showed that 80 (65.5%, P<0.001) of the patients in the older adult group had coexisting hypertension, and the number of patients who received pre-operative chemotherapy was significantly lower (P<0.001) (
Table 1). Preoperative CONUT values were not significantly different between the two groups, but preoperative PNI was 43.0±5.7 in the older adult group, which was significantly lower (P=0.032). There was no difference in stage between the two groups. On the other hand, DP was performed significantly higher in the older adult group than in the non-older adult group (P=0.007), with total pancreatectomy (TP) being less common in the older adult group. In addition, 33 patients underwent combined portal vein resection (27.1%, P<0.001), and operative time and blood loss were also significantly lower than patients in the non-older adult group (P<0.001 for each).
When examining the postoperative course, there was no difference in overall postoperative complications or major complications, and postoperative pancreatic fistula tended to be more common in the older adults, but with no significant difference (P=0.058) (
Table 2).
Postoperative pneumonia occurred in 13 patients in the older adult group (10.6%), which was significantly higher than that in the non-older adult group (P=0.02). Long-term prognosis showed that overall survival was significantly lower in the older adult group than in the non-older adult (P=0.002) (
Fig. 2A). However, there was no significant difference in recurrence-free survival (
Fig. 2B).
Nutritional disorders were defined in 36 (29.5%) of the 122 older adult patients using PNI, and 31 (25.4%) were identified by CONUT values. Comparing the cases of pancreatic cancer resection in the older adult group between PNI 40 or less and the other groups, there was no difference in background factors, but there were seven cases of preoperative chemotherapy in the PNI 40 or less group (19.4%), which was significantly less (P=0.022) (
Table 3). Although postoperative outcomes were similar, mortality after pancreatectomy was significantly higher in the PNI 40 or less group, with three (8.3%) deaths in the hospital (P=0.042) (
Table 4).
On the other hand, in the CONUT classification, the group with nutritional disorders did not differ from the group without nutritional disorders in terms of background factors (
Table 5). However, as with the PNI classification, postoperative mortality was significantly higher in patients with nutritional disorder (P<0.001) (
Table 6). The four deaths among older adult patients were all due to infectious complications, except for one death due to primary disease, but no other trends were observed (
Table 7). In these patients, there were one case of high intraoperative blood loss due to invasive surgery including portal vein and celiac axis resection, and two cases of postoperative pancreatic fistula, which resulted in infectious complications.
Discussion
Key results
Older adult patients with pancreatic cancer after resection had more hypertension (65.5%) and received less preoperative chemotherapy than non-older adult patients. Preoperative PNI was lower (43.0±5.7), but CONUT values were similar. Surgeries were less extensive, with shorter duration and less blood loss. Postoperative pneumonia incidence and overall mortality were significantly higher among older adult patients, though recurrence-free survival was similar. Nutritional disorders, defined by low PNI or CONUT values, significantly correlated with increased postoperative mortality, primarily due to infectious complications, including pancreatic fistula.
Interpretation/comparison with previous studies
Pancreatectomy, including PD and TP, is a difficult and highly invasive procedure that requires careful management in older adult patients. The risk of pancreatic fistula is particularly high in PD, and the incidence of postoperative complications and mortality rates are reported to be 41.6% and 2.8%, respectively, even with the improvement of surgical techniques and the development of perioperative management [
8]. However, there are an increasing number of reports in recent years showing that PD for the older adult has comparable postoperative outcomes to those for younger patients [
9,
10]. In this study, we compared the postoperative results between older adult and non-older adult patients, and found that pancreatectomy can be performed safely in older adult patients as in previous reports. However, the older adult patients had lower preoperative PNI, more nutritional problems, and more preoperative comorbidities. On the other hand, the perioperative results showed that the postoperative outcome of the older adult patients was relatively good, even if they had preoperative nutritional problems. Although the influence of preoperative patient selection is undeniable, it is also possible that the incidence of postoperative complications was reduced by shifting to less invasive procedures and by efforts to reduce blood loss and operation time. Even so, not all complications were controlled, and postoperative pneumonia was more common in the older adult group.
The incidence of postoperative pneumonia in the older adult was significantly higher than that in the non-older adult group. Prevention of postoperative pneumonia in the older adult requires not only reduction of surgical invasiveness but also more multifaceted medical care. The effectiveness of oral care in reducing postoperative infectious complications after PD surgery [
11] and the introduction of a perioperative management team in preventing pneumonia [
12] have been reported, suggesting that there is room for further improvement in the prevention of postoperative pneumonia in older adult patients with pancreatic cancer.
In a study of long-term prognosis in older adult patients with pancreatic cancer, overall survival was significantly lower than that in non-older adult patients, but recurrence-free survival was similar. Although it is difficult to make a generalized statement because the study did not match the surgical technique and stage, the overall survival rate was probably influenced by the median age (78 years) and comorbidities in the older adult group. On the other hand, there was no difference in recurrence-free survival or R0 resection rate, suggesting that surgical resection for pancreatic cancer in the older adult is comparable to that in the non-older adults. In addition, it is interesting to note that preoperative chemotherapy was administered at a significantly lower rate in older adult patients with pancreatic cancer. Currently, the standard treatment for resectable pancreatic cancer is pre-operative chemotherapy with gemcitabine plus S1 and surgical resection, but the PREP-02/JSAP-05 trial, on which this standard is based, did not enroll patients aged 80 or older [
13]. Although the long-term prognosis was not examined in our study, 39 (31.9%) of the patients in the late-stage older adult group were aged 80 years or older.
Considering that the outcomes of resected patients are similar, it is possible that preoperative chemotherapy is unnecessary for patients over 80 years of age. The necessity of preoperative treatment for pancreatic cancer patients over 80 years of age should also be considered in the future. In addition, among resected pancreatic cancer patients in the older adults, significantly more patients with a PNI of 40 or less did not receive preoperative chemotherapy. Although our institution does not conduct nutritional assessment as a preoperative treatment criterion for pancreatic cancer, it is possible that patients were selected a priori based on nutritional assessment. In this sense, the significance of nutritional evaluation as a requirement for preoperative treatment of pancreatic cancer in the older adults may be significant.
In older adult patients with pancreatic cancer, preoperative nutritional disorders were considered a risk factor for post-operative hospital mortality, although they did not affect other complications. Ishida et al. [
14] compared preoperative nutritional status and postoperative complications in PD and reported that postoperative complications were significantly more frequent in patients with preoperative nutritional problems than in normal patients when the effect of pancreatic fistula was excluded. Older adult patients have a decline in immune function associated with aging, and aging has been cited as a poor prognostic factor in patients with sepsis [
1]. Yanagawa et al. [
15] also studied gastric cancer patients with pyloric stenosis, and reported that poor preoperative nutrition was associated with a high risk of postoperative infectious complications. In this report, three out of four patients who died in the hospital also had infectious complications, suggesting that older adult patients with preoperative malnutrition who underwent pancreatic cancer resection are more prone to infectious complications and more likely to develop serious complications.
It is also interesting to note that in this comparison between older adult and non-older adult patients, the older adult patients had significantly lower PNI, whereas no difference was observed in CONUT scores. Although both PNI and CONUT included albumin and total lymphocyte counts as calculation factors, total cholesterol, which is considered an indicator of lipid metabolism, was included only in CONUT. Nutritional improvement has been reported by administering pancrelipase to patients with pancreatic exocrine insufficiency [
16], and the effect on pancreatic cancer patients may be equivalent to that of pancrelipase. Early administration of pancrelipase in pancreatic cancer patients may improve preoperative nutritional status and postoperative outcomes.
It was a single-center, retrospective study and that surgical treatment was likely to have been performed only in selected patients with older adult disease. In addition, we did not include any nutritional indices such as muscle mass, performance status, hypertension, and diabetes mellitus in this study. However, it is also true that a simpler and more objective evaluation index is required in daily clinical practice, and the development of a more versatile index is expected in the future.
Conclusion
We examined cases of pancreatic cancer resection in the older adults, and found that surgical treatment was safe and less invasive, although many patients with pancreatic cancer in the older adults were accompanied by nutritional disorders. However, preoperative malnutrition is a risk factor for in-hospital mortality, and it is necessary to take measures such as improving malnutrition and avoiding over-invasive surgery.
Authors’ contribution
Conceptualization: TM. Data curation: TM, MI. Formal analysis: TM, MI. Investigation: TM, MI. Methodology: TM, MI. Project administration: TM. Supervision: MU. Funding acquisition: Not applicable. Writing – original draft: TM. Writing – review & editing: MI, MM, KN, TK, MU. All authors read and approved the final manuscript.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgements
None.
Supplementary materials
None.
Fig. 1.CONUT value calculation table.
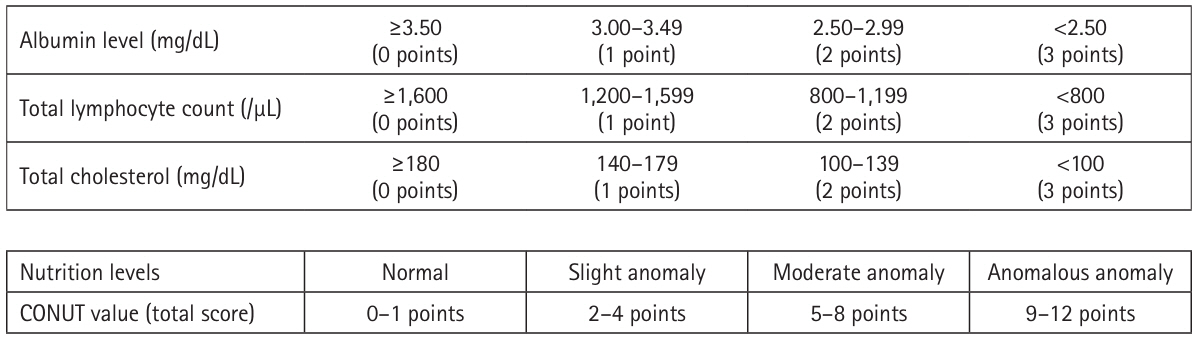
Fig. 2.Long-term outcomes after pancreatic cancer resection in older adult (≥75 yeasr) and non-older (<75 years) patients. Comparing patients aged ≥75 years with those aged <75 years, the overall survival rate was significantly better in patients aged <75 years (P=0.002) (A). However, the two groups had no significant difference in recurrence-free survival (P=0.198) (B).
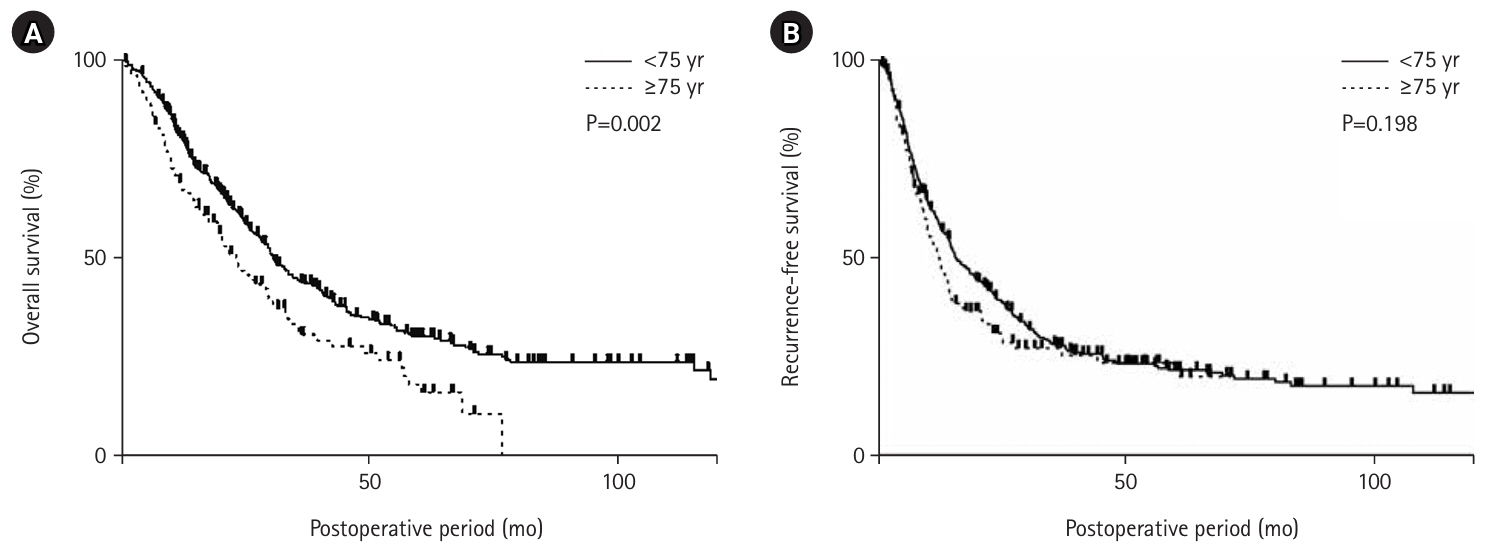
Table 1.Comparison of the background and intraoperative factors between the elderly and non-elderly patients
|
|
<75 yr |
≥75 yr |
P-value |
|
Sex (male:female) |
|
253:174 |
69:53 |
0.594 |
|
Preoperative CONUT value |
|
2 (0-11) |
3 (0-9) |
0.260 |
|
Preoperative PNI |
|
44.3±6.0 |
43.0±5.7 |
0.032a
|
|
Diabetes mellitus |
|
220 (51.5) |
61 (50.0) |
0.837 |
|
Hypertension |
|
199 (46.6) |
80 (65.5) |
<0.001a
|
|
Preoperative chemotherapy |
|
230 (53.8) |
43 (35.3) |
<0.001a
|
|
Stage of an illness |
0 |
3 (0.7) |
2 (1.6) |
0.070 |
|
IA |
29 (6.8) |
8 (6.6) |
|
|
IB |
7 (1.6) |
1 (0.8) |
|
|
IIA |
98 (22.9) |
43 (35.2) |
|
|
IIB |
233 (54.6) |
60 (49.2) |
|
|
III |
5 (1.2) |
0 |
|
|
IV |
52 (12.2) |
8 (6.6) |
|
|
Operative procedure |
PD |
248 (58.1) |
61 (50.0) |
|
|
DP |
118 (27.6) |
51 (41.8) |
0.007a
|
|
TP |
61 (14.3) |
10 (8.2) |
|
|
Combined portal vein resection |
|
172 (40.3) |
33 (27.1) |
0.008a
|
|
Operation time (min) |
|
534 (150–1,160) |
481 (182–851) |
<0.001a
|
|
Amount of blood loss (mL) |
|
1,179 (22–7,250) |
906 (63–9,695) |
<0.001a
|
|
R0 resection |
|
358 (83.8) |
100 (82.0) |
0.597 |
Table 2.Comparison of postoperative results between resected pancreatic cancer cases in elderly and non-elderly patients
|
<75 yr |
≥75 yr |
P-value |
|
Postoperative hospital stay (day) |
24 (5–193) |
25 (3–415) |
0.858 |
|
Total complications |
328 (76.8) |
87 (71.3) |
0.232 |
|
Serious complications |
121 (28.3) |
36 (29.5) |
0.820 |
|
Death in hospital |
11 (2.5) |
4 (3.2) |
0.752 |
|
Readmission within 30 day |
19 (15.5) |
54 (12.6) |
0.613 |
|
SSI |
106 (24.8) |
36 (29.5) |
0.294 |
|
Organ space SSI |
71 (16.6) |
24 (19.6) |
0.419 |
|
Postoperative pancreatic fistula |
56 (13.1) |
25 (20.4) |
0.058 |
|
Postoperative pneumonia |
21 (4.9) |
13 (10.6) |
0.020a
|
|
Septicemia |
26 (6.0) |
6 (4.9) |
0.826 |
|
Thrombosis |
22 (5.7) |
7 (5.1) |
0.818 |
Table 3.Background and intraoperative factors of late-stage elderly pancreatic cancer cases grouped by PNI40
|
|
PNI≤40 |
PNI>40 |
P-value |
|
Sex (male:female) |
|
24:12 |
45:41 |
0.165 |
|
Hypertension |
|
26 (72.2) |
54 (62.8) |
0.404 |
|
Diabetes mellitus |
|
19 (52.8) |
42 (48.8) |
0.842 |
|
Preoperative chemotherapy |
|
7 (19.4) |
36 (41.8) |
0.022a
|
|
Operative procedure |
PD |
21 (58.3) |
40 (46.5) |
0.536 |
|
DP |
14 (38.9) |
37 (43.0) |
|
|
TP |
1 (2.8) |
9 (10.5) |
|
|
Combined portal vein resection |
|
12 (33.3) |
21 (24.4) |
0.372 |
|
Operation time (min) |
|
502 (182–851) |
470 (202–845) |
0.306 |
|
Amount of blood loss (mL) |
|
1,222 (82–9,639) |
834 (63–9,695) |
0.149 |
|
R0 resection |
|
28 (77.8) |
72 (83.7) |
0.529 |
Table 4.Postoperative outcomes of older adult patients with pancreatic cancer grouped by PNI40
|
PNI≤40 |
PNI>40 |
P-value |
|
Postoperative hospital stay (day) |
27.5 (3–163) |
24 (10–415) |
0.355 |
|
Total complications |
28 (77.8) |
59 (68.6) |
0.382 |
|
Serious complications |
14 (38.9) |
22 (25.6) |
0.191 |
|
Death in hospital |
3 (8.3) |
1 (1.2) |
0.042a
|
|
Readmission within 30 day |
3 (8.3) |
16 (18.6) |
0.181 |
|
SSI |
12 (33.3) |
24 (27.9) |
0.663 |
|
Organ space SSI |
8 (22.2) |
16 (18.6) |
0.627 |
|
Postoperative pancreatic fistula |
7 (19.4) |
18 (20.9) |
0.852 |
|
Postoperative pneumonia |
6 (16.1) |
7 (8.1) |
0.200 |
|
Septicemia |
1 (2.7) |
5 (5.8) |
0.669 |
|
Thrombosis |
4 (11.1) |
3 (3.5) |
0.193 |
Table 5.Background and intraoperative factors of older adult patients with pancreatic cancer grouped by CONUT values
|
|
No nutritional disorders |
Nutritional disorders |
P-value |
|
Sex (male:female) |
|
48:43 |
21:10 |
0.207 |
|
Hypertension |
|
21 (67.7) |
59 (64.8) |
0.829 |
|
Diabetes mellitus |
|
17 (54.8) |
44 (48.3) |
0.677 |
|
Preoperative chemotherapy |
|
35 (38.5) |
8 (25.8) |
0.276 |
|
Operative procedure |
PD |
43 (47.2) |
18 (58.1) |
0.388 |
|
DP |
39 (42.9) |
12 (38.7) |
|
|
TP |
9 (9.9) |
1 (3.2) |
|
|
Combined portal vein resection |
|
24 (26.4) |
9 (29.0) |
0.816 |
|
Operation time (min) |
|
471 (202–845) |
498 (182–851) |
0.462 |
|
Amount of blood loss (mL) |
|
870 (63–9,695) |
1,145 (82–9,639) |
0.432 |
|
R0 resection |
|
76 (83.5) |
24 (77.4) |
0.565 |
Table 6.Postoperative outcomes of older adult patients with pancreatic cancer grouped by CONUT value
|
No nutritional disorders |
Nutritional disorders |
P-value |
|
Postoperative hospital stay (day) |
24 (10–415) |
28 (3–100) |
0.085 |
|
Total complications |
61 (67.0) |
26 (83.8) |
0.106 |
|
Serious complications |
25 (27.5) |
11 (35.5) |
0.494 |
|
Death in hospital |
0 |
4 (12.9) |
<0.001a
|
|
Readmission within 30 day |
17 (18.7) |
2 (6.5) |
0.151 |
|
SSI |
25 (27.4) |
11 (35.5) |
0.494 |
|
Organ space SSI |
18 (19.8) |
6 (19.4) |
0.958 |
|
Postoperative pancreatic fistula |
20 (21.9) |
5 (16.1) |
0.799 |
|
Postoperative pneumonia |
8 (8.8) |
5 (16.1) |
0.312 |
|
Septicemia |
4 (4.4) |
2 (6.4) |
0.643 |
|
Thrombosis |
5 (5.5) |
2 (6.5) |
0.845 |
Table 7.Pancreatic cancer in-hospital deaths in older adult patients
|
Age |
Sex |
Technique |
PVR |
Blood loss (mL) |
Operation time (min) |
Postoperative pancreatic fistula |
PNI |
CONUT |
Cause of death |
|
1 |
76 |
Male |
DP |
None |
9,639 |
453 |
Yes |
37.1 |
5 |
Renal failure, pneumonia |
|
2 |
75 |
Male |
DP-CAR |
None |
350 |
535 |
None |
41.4 |
5 |
Pancreatic cancer liver metastasis |
|
3 |
79 |
Women |
SSPPD |
None |
545 |
531 |
Yes |
29.7 |
8 |
Sepsis |
|
4 |
82 |
Male |
SSPPD, right colon resection |
Yes |
1,483 |
529 |
None |
36.7 |
6 |
Sepsis, ARDS |
References
- 1. Inoue S. Classification of postoperative organ failure. JSSEM 2021;55:6-10. ArticlePubMed
- 2. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. ArticlePubMed
- 3. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. J Jpn Surg Soc 1984;85:1001-1005.ArticlePDF
- 4. Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, et al. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 2014;18:1108-15. ArticlePubMedPDF
- 5. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. ArticlePubMed
- 6. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584-91. ArticlePubMed
- 7. Japan Pancreas Society. Classification of pancreatic carcinoma. Kanehara & Co., Ltd.; 2016.ArticlePDF
- 8. Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci 2017;24:243-51. ArticlePubMedPMCPDF
- 9. Sugawara S, Watanabe T, Tezuka K, Hirai I, Kimura W. Surgical outcomes of pancreaticoduodenectomy and postoperative changes in nutritional indexes in elderly patients over 75. Nihon Rinsho Geka Gakkai Zasshi 2018;79:267-72. Article
- 10. Murakami M, Shimizu J, Koga C, Hitora T, Kawabata R, Oda N, et al. Analysis of pancreaticoduodenectomy for elderly patients aged 75 years or older. Gan To Kagaku Ryoho 2015;42:2351-3. ArticlePubMed
- 11. Nobuhara H, Shinji Y, Ito K, Itamoto T. Preventive effect of perioperative oral care on postoperative infectious complications of gastrointestinal cancer. JSSEM 2017;51:165-74. ArticlePubMed
- 12. Okamura A, Watanabe M, Imamura Y, Kamiya S, Hayami M, Yamashita K, et al. Current status of postoperative recurrent nerve palsy after thoracoscopic esophagectomy and significance of perioperative management team in prevention of postoperative pneumonia. Jpn J Tracheoesophageal Sci 2017;68:189-91. Article
- 13. Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 2019;49:190-4. ArticlePubMed
- 14. Ishida M, Motoi F, Sato H, Murakami M, Maeda S, Kudoh K, et al. Preoperative nutritional assessment by CONUT (Controlling Nutritional Status) and postoperative complications after pancreatoduodenectomy. J JSPEN 2018;33:641-6. Article
- 15. Yanagawa T, Iwazawa T, Fujita J, Kawanishi K, Kawanishi K, Nagai K, et al. Preoperative nutritional status and postoperative infectious complications in gastric cancer patients with pyloric stenosis. JSSEM 2013;47:139-45. ArticlePubMed
- 16. Miyoshi H, Inui W, Yamamoto T, Noda A, Nakazawa T, Hayashi K, et al. Short-term therapeutic effect of pancrelipase in patients with chronic pancreatitis. J Hepatobiliary Pancreat Cancer Res 2018;15:27-32.Article

 , Masaharu Ishida
, Masaharu Ishida , Masamichi Mizuma
, Masamichi Mizuma , Kei Nakagawa
, Kei Nakagawa , Takashi Kamei
, Takashi Kamei , Michiaki Unno
, Michiaki Unno



 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

