Abstract
-
Purpose
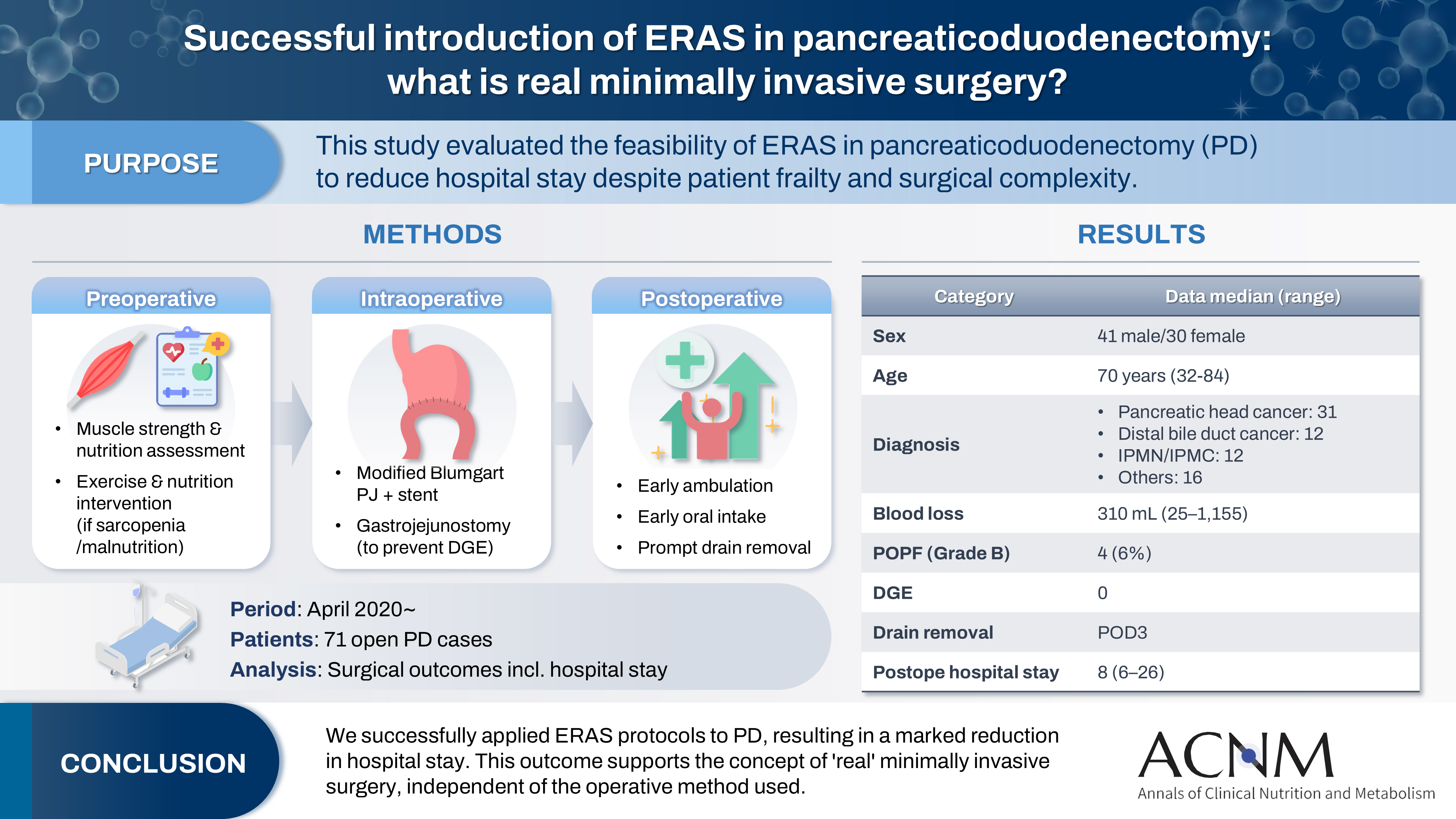
The introduction of Enhanced Recovery After Surgery (ERAS) protocols for pancreaticoduodenectomy (PD) has been considered challenging due to factors such as preexisting malnutrition, sarcopenia, the complexity of the surgery, and the high incidence of postoperative complications, including postoperative pancreatic fistula (POPF) and delayed gastric emptying (DGE). This study aimed to determine whether ERAS could be implemented in PD to achieve shorter postoperative hospital stays.
-
Methods
Our novel approach consists of three components. Preoperatively, we routinely assess patients' muscle strength and nutritional status and initiate exercise and nutritional interventions for those identified with sarcopenia or malnutrition. Intraoperatively, we perform pancreaticojejunostomy using a modified Blumgart’s technique with our stent placement policy and utilize new gastrojejunostomy methods to prevent DGE. Principles of postoperative management are early ambulation, early oral intake, and early drain removal. Since April 2020, we have employed this strategy and retrospectively evaluated its effectiveness. We enrolled 71 consecutive patients who underwent open PD with curative intent. Various surgical outcomes, including postoperative hospital stay, were analyzed.
-
Results
There were 41 men and 30 women, with a median age of 70 years. Preoperative diagnoses included pancreatic head cancer in 31, distal bile duct cancer in 12, and others. Median intraoperative blood loss was 310 mL. Grade B POPF occurred in four patients (6%). No cases of DGE were observed. The median postoperative hospital stay was 8 days (range, 6–26 days).
-
Conclusion
We successfully implemented ERAS protocols in PD and achieved a significantly reduced postoperative hospital stay. We propose that this approach is “real minimally invasive surgery," regardless of the surgical technique used.
-
Keywords: Enhanced Recovery After Surgery; Length of stay; Minimally invasive surgery; Pancreaticoduodenectomy; Sarcopenia
Graphical abstract
Introduction
Pancreaticoduodenectomy (PD) is a standard procedure for pancreatic head cancer, but it is highly invasive and is associated with a substantial risk of complications such as pancreaticojejunostomy or gastrojejunostomy leakage, biliary-enteric anastomosis leakage, delayed gastric emptying (DGE), and intra-abdominal abscess, all of which contribute to prolonged postoperative hospital stays. Patients scheduled for PD frequently present with preoperative malnutrition, weight loss, diabetes, obstructive jaundice, and sarcopenia. With the advent of a super-aged society, there has been a notable rise in the number of octogenarians undergoing surgery for pancreatic cancer.
Furthermore, based on the findings of the Prep-02/JSAP-5 trial [
1], the 2019 pancreatic cancer guidelines now recommend neoadjuvant therapy even for resectable pancreatic cancer, albeit with a weak recommendation [
2]. As a result, preoperative therapy has become widely adopted for all pancreatic cancer cases. Therefore, comprehensive perioperative management—including the period during preoperative therapy, particularly focusing on exercise and nutritional management—is essential for improving short-term outcomes.
In recent years, Enhanced Recovery After Surgery (ERAS) programs have been actively implemented in the context of pancreatic resection [
3]. Studies have shown that ERAS protocols can shorten postoperative hospital stays, facilitate earlier discharge, and reduce the overall rate of complications [
4-
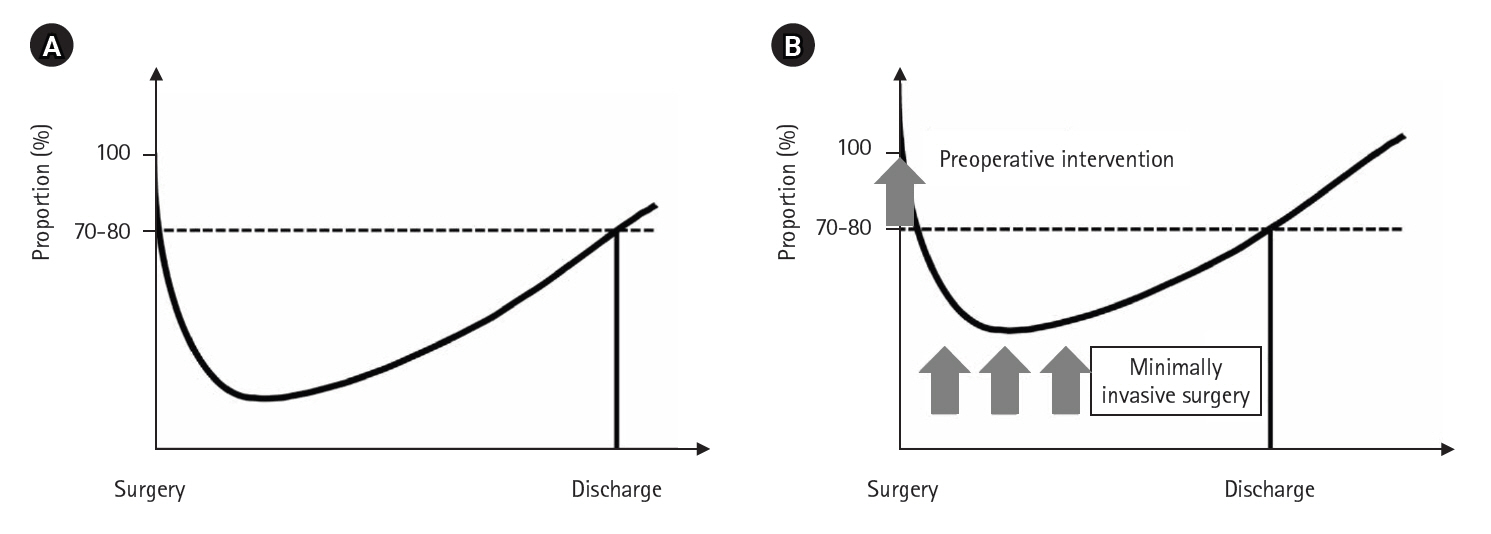
7]. However, early discharge may refer to discharge home or transfer to another facility, but only discharge to home reflects true recovery. Conceptually, if a postoperative recovery curve is drawn with the patient’s baseline physical condition at 100% at surgery, the curve declines postoperatively and then improves, with discharge typically occurring when the patient achieves 70% to 80% recovery (
Fig. 1A). How can early discharge be achieved? The answer is simple: by shifting the postoperative recovery curve upward, the intersection with the 70–80% line moves to the left (
Fig. 1B). Key prerequisites for this include preoperative interventions to improve the patient’s overall condition, minimally invasive surgery to reduce postoperative stress, and rigorous implementation of ERAS protocols.
Perioperative management of PD at St. Luke’s International Hospital
Traditionally, Japanese surgeons have widely believed that postoperative hospital stays exceeding 3 weeks are inevitable following PD. Recently, “minimally invasive” surgical procedures such as laparoscopic and robot-assisted procedures have been introduced for PD, yet these innovations have not substantially reduced postoperative hospital stays. Since my appointment at this hospital in October 2019, we have observed a gradual decrease in the length of stay following PD, with some patients discharged in approximately 10 days. This experience prompted a reevaluation of the conventional belief that the postoperative hospital stay after PD is always over 3 weeks, leading us to actively incorporate the principles of ERAS.
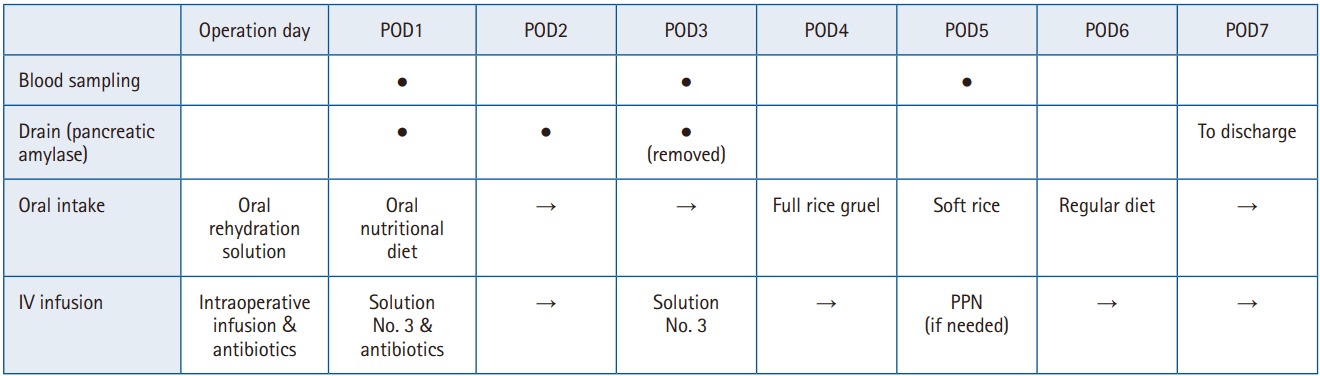
In April 2020, we established and began operating a clinical pathway aimed at “discharge to home within 10 days after PD.” Here, we outline our perioperative management strategy for PD (
Fig. 2). The items and numbers in parentheses refer to the most recent 2019 ERAS Society guidelines for perioperative care in PD [
3].
Preoperative management
For patients scheduled to undergo hepatobiliary and pancreatic surgery, including PD, we assess grip strength and nutritional status in addition to standard preoperative evaluations. We then provide preoperative exercise and nutritional interventions as needed. At our institution, except for patients with uncontrolled diabetes, most are admitted the day before surgery, which limits opportunities for inpatient preoperative rehabilitation. To address this, as part of preoperative counseling, outpatient nurses provide all surgical candidates with a comprehensive booklet detailing respiratory muscle training, resistance exercise, and aerobic exercise routines as “home rehabilitation,” which patients are instructed to perform prior to surgery (1. Preoperative counseling, 2. Prehabilitation).
Recently, both European and Asian sarcopenia working groups have established new diagnostic algorithms [
8,
9], both of which begin with muscle strength assessment and, if muscle strength is reduced, proceed to evaluate physical function and skeletal muscle mass/quality to confirm sarcopenia or severe sarcopenia. Grip strength is a simple and cost-effective measure in the outpatient setting. Previous research at my former institution demonstrated a strong correlation between preoperative skeletal muscle mass and grip strength in living-donor liver transplantation cases [
10], supporting grip strength as a surrogate for skeletal muscle mass. Therefore, in addition to “home rehabilitation,” patients with reduced grip strength (defined by Asian Working Group for Sarcopenia criteria: <28 kg for men, <18 kg for women [
9]) undergo exercise therapy supervised by a physical therapist. All surgical candidates also visit the perioperative care center, where anesthesiologists and pharmacists provide comprehensive assessments, video explanations of preoperative preparation, and instructions regarding medication discontinuation, as well as guidance on smoking and alcohol cessation (1. Preoperative counseling, 4. Smoking and alcohol cessation).
For nutritional assessment, among several available parameters, we prioritize prealbumin (transthyretin), a protein with a rapid turnover rate, and zinc. While the normal value for prealbumin is ≥22 mg/dL, we set a pragmatic target of ≥15 mg/dL. Patients with prealbumin below 15 mg/dL receive nutritional supplementation and guidance from registered dietitians (5. Preoperative nutritional management). Zinc is a critical trace element for taste, immune function, wound healing, glucose metabolism, and antioxidant effects, and it plays an important role in early postoperative recovery. Blood zinc levels transiently decrease after various gastrointestinal operations, including gastrectomy, colectomy, hepatectomy, PD, and liver transplantation [
11,
12]. Recently, Iseki et al. [
13] identified preoperative hypozincemia as an independent risk factor for postoperative infectious complications after pancreatic resection, including PD. Therefore, hypozincemia may contribute to complications such as pancreatic fistula, poor healing of the pancreatic stump, intra-abdominal abscess, poor oral intake, and impaired glucose tolerance. Accordingly, we routinely check zinc levels at outpatient visits, and for patients with hypozincemia (<80 μg/dL), we prescribe zinc acetate (Nobelzin, 50–100 mg in two divided doses). Preoperative immunonutrition is not performed (6. No preoperative immunonutrition).
Historically, upfront surgery was the standard for resectable pancreatic cancer, limiting the window for preoperative rehabilitation and nutritional intervention. However, in recent years, the usefulness of neoadjuvant therapy for resectable pancreatic cancer has been reported [
1], and the pancreatic cancer treatment guidelines now recommend neoadjuvant therapy even for resectable cases [
2]. This provides an opportunity to use the treatment period (two cycles of gemcitabine hydrochloride plus S-1 over 6 weeks, plus rest) for targeted preoperative interventions, making exercise and nutritional optimization more feasible in pancreatic surgery.
Additionally, patients are instructed to consume oral rehydration solutions from the night before until the morning of surgery to reduce insulin resistance (7. Shortened preoperative fasting and carbohydrate loading).
Intraoperative management
Antibiotics (primarily second-generation cephalosporins) are administered within 60 minutes prior to incision (10. Prophylactic antibiotic administration). Pre-anesthetic medication is omitted (8. No pre-anesthetic medication), and all cases are managed with epidural anesthesia (11. Epidural anesthesia).
Intraoperatively, meticulous handling of the pancreas, secure pancreaticojejunostomy to minimize the risk of postoperative pancreatic fistula, vigilant measures to prevent contamination of the surgical field with bile, and thorough intra-abdominal lavage are all rigorously practiced to prevent postoperative infection. Preventing DGE—a complication that prolongs fasting—also necessitates precise gastric-jejunal anastomosis techniques. As the focus of this article is ERAS, detailed surgical techniques are described elsewhere [
14]. To prevent intestinal edema, anesthesiologists are instructed to carefully regulate intraoperative fluid administration (18. Fluid balance). Although not encountered in this cohort, intraoperative feeding jejunostomy tube placement may be considered in cases where early postoperative oral intake is anticipated to be difficult. Nasogastric tubes are routinely removed in the operating room (17. No nasogastric tube placement).
After PD, all patients are admitted to the intensive care unit (ICU). Based on a risk assessment, additional measures such as deep vein thrombosis prevention (using unfractionated heparin, low-molecular-weight heparin, or intermittent pneumatic compression) (9. Thromboprophylaxis) and prophylaxis for postoperative nausea and vomiting (14. Prevention of postoperative nausea and vomiting) are implemented as appropriate.
Postoperative management
Postoperative exercise and nutritional management are of paramount importance. The fundamental principles of postoperative care include early mobilization, early initiation of oral intake, and prompt removal of drains.
After surgery, patients are susceptible to a rapid decline in skeletal muscle mass due to surgical stress, pain, and immobilization from intravenous lines and drains. Skeletal muscle mass decreases by more than 2% after surgery [
10], whereas normal aging causes an annual decline of only about 1%. Thus, in just 1 week after surgery, patients experience muscle loss equivalent to over 2 years of aging. Therefore, starting at 9:00 a.m. on postoperative day 1 (POD1), all PD patients, who are managed in the ICU postoperatively, begin ambulation with a physical therapist. After transferring to the general ward at noon that same day, patients continue walking in the ward corridors under therapist supervision to further prevent muscle wasting (25. Early and planned mobilization).
Nutritional management is similarly structured and multidisciplinary. At 7:30 a.m. on POD1, a nutrition care plan is formulated during an ICU round that includes dietitians, physical therapists, and nurses, emphasizing team-based collaboration. Intravenous fluids are commenced on the morning of POD1, while nutritional supplements containing collagen peptides and zinc are introduced at lunchtime. Previously, a postoperative Gastrografin study was conducted on POD3 to evaluate passage at the gastrojejunostomy, but due to intraoperative improvements, DGE is no longer seen and this step has recently been omitted. Starting on POD4, oral intake with meals (rice porridge) is initiated, and a high-potency pancrelipase preparation (Lipacreon 1,800 mg/day) is given immediately after meals. Diet advancement is individualized based on patient tolerance and preferences.
Intravenous fluid management from POD1 consists of maintenance fluids (1,500–2,000 mL/day), and central venous nutrition is not used. For patients with inadequate oral intake after meals are started, peripheral parenteral nutrition containing fat (Enefluid) is administered, avoiding the need for central venous nutrition.
Drains (two: one placed in the foramen of Winslow and one dorsal to the pancreaticojejunostomy) are monitored daily for amylase content starting POD1. Drains are removed when the amylase level is ≤1,000 U/L (typically on POD3) or the amount of drain discharge is <20 mL (19. Early drain removal). Somatostatin analogs are not utilized (20. No somatostatin analogs).
Blood glucose levels are checked four times daily for glycemic management, and glucose control is achieved in collaboration with endocrinologists, utilizing a sliding scale or long-acting insulin as appropriate (16. Postoperative glycemic control).
Postoperative antibiotics are generally given until POD2, and are continued or adjusted according to POD3 laboratory data if intra-abdominal infection is suspected.
On POD6, a computed tomography scan is performed, and if there are no particular problems, normal white blood cell count, and no fever, the patient can consume at least half of their meals and go to the toilet independently; discharge to home is permitted from POD7 onward. The discharge date is determined according to the patient's wishes and family circumstances.
We believe that, in the absence of surgical complications, “PD=DG (distal gastrectomy)”—meaning such straightforward perioperative management is achievable through a collaborative team-based approach involving surgeons, internists, and other healthcare professionals.
Validation of appropriateness
We assessed the appropriateness of our perioperative management approach in 71 consecutive patients who underwent PD with curative intent at our institution after April 2020. Parameters examined included age, sex, primary disease, operative time, blood loss, pancreatic texture, timing of first ambulation and diet initiation, postoperative complications, timing of drain removal, postoperative length of stay, and readmission within 2 weeks of discharge (27. Audit).
Table 1 summarizes patient characteristics and outcomes. The median age was 70 years, with 11 patients (15%) aged over 80. Despite a median operative time of 473 minutes, reflecting a meticulous surgical approach, the median blood loss was relatively low at 310 mL, with a recent increase in cases under 100 mL. Drains were typically removed on POD3, and diets were initiated on POD3 or 4. Grade B pancreatic fistula developed in four cases (6%), but there were no instances of DGE. As a result, the median postoperative hospital stay was 8 days (minimum 6 days), and all patients were discharged home. There were five readmissions (7%) within 2 weeks, including two for poor oral intake (one following PD after total gastrectomy), one for intra-abdominal abscess, one for acute cholangitis, and one for COVID-19 infection. Excluding the COVID-19 case, the readmission rate was 6%, which we consider acceptable.
A domestic report from Japan, where healthcare systems are relatively uniform, described the introduction of ERAS in PD (48 cases) by Teramura et al. [
15], which did not significantly reduce postoperative hospital stay—17 days after ERAS introduction versus 18 days prior. The lack of improvement may relate to the high rates of postoperative complications, particularly pancreatic fistula and DGE (39.6%), and Clavien-Dindo grade 3 or higher complications (20.8%). Conversely, Takagi et al. [
6] reported a significant reduction in mean postoperative hospital stay (20.1 days after ERAS implementation vs. 26.9 days prior) in 37 cases. Although their Clavien-Dindo grade 3 or higher complication rate after ERAS remained 32.4% (56.8% prior to ERAS). These findings suggest that to achieve early discharge with ERAS, it is critical not only to optimize perioperative management but also to continually refine surgical techniques to reduce postoperative complications.
Conclusion
We have described our strategy for “perioperative metabolic and nutritional management for PD with early discharge as the target outcome” at our institution. Through a range of initiatives—including implementation of ERAS protocols, multidisciplinary team-based care, and meticulous surgical technique aimed at minimizing complications—we have successfully achieved earlier postoperative recovery and discharge. Although the sample size is currently limited and further validation is required, our median postoperative hospital stay following PD has been reduced from over 3 weeks to just 8 days. Early discharge benefits patients by reducing both physical and economic burdens.
Recently, laparoscopic and robot-assisted surgery have gained recognition as minimally invasive techniques and is being widely adopted in various fields; we are also incorporating these methods in our practice. However, it is important to question whether laparoscopic/robot-assisted surgery is truly minimally invasive surgery. These are merely techniques and should not be seen as ultimate goals. If the focus shifts to the method rather than the patient’s outcome, unnecessarily prolonged operative times or increased complications can result. Therefore, whether laparoscopic, robot-assisted, or open, the optimal surgical approach should be selected for each patient based on general condition, tumor location, tumor size, and surgeons' skill, with the aim of careful, complication-free surgery and proper perioperative management as detailed in this report. Regardless of approach, this philosophy embodies “true minimally invasive surgery.”
Lastly, I would like to stress that our short postoperative hospital stay in this cohort is not aim but outcome. We should not pursue only short postoperative hospital stay. Our aim is to continue refining our surgical practice and perioperative management in order to consistently achieve early discharge and optimal outcomes for patients.
Authors’ contribution
Conceptualization: TK, YM. Data curation: TK, YM. Methodology/formal analysis/validation: TK, YM. Project administration: all authors. Writing–original draft: TK. Writing–review & editing: all authors. All authors read and approved the final manuscript.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.
Supplementary materials
None.
Correction
This article was corrected on November 13, 2025, to revise the article type.
Fig. 1.Postoperative recovery curve (A) and postoperative recovery curve allowing early discharge (B).
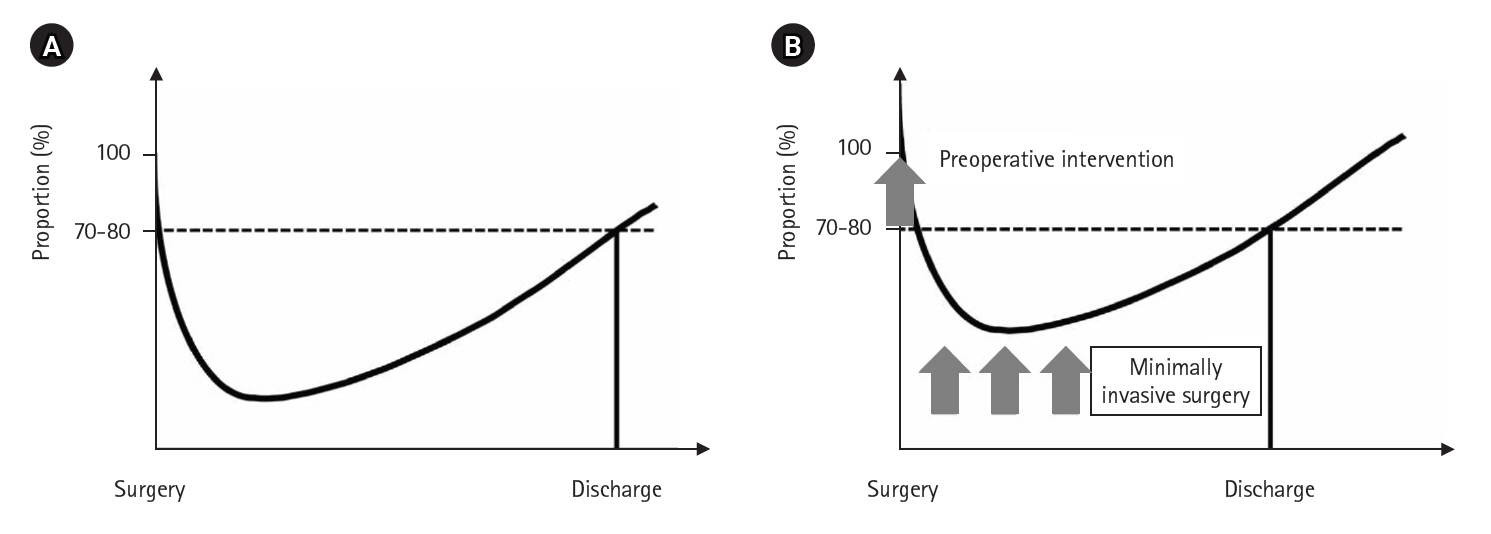
Fig. 2.Postoperative management after pancreaticoduodenectomy (key points only). POD, postoperative day; IV, intravenous; PPN, peripheral parenteral nutrition. ● indicates the procedure was performed, and → indicates continuation from the previous day.
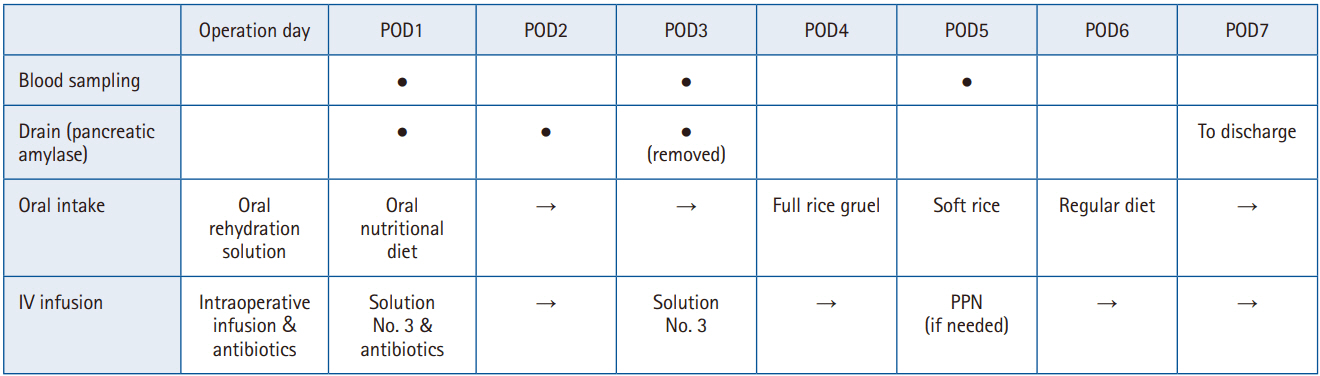
Table 1.Patient background and outcomes for 71 consecutive cases of pancreaticoduodenectomy
|
Variable |
Median (range) or No. (%) |
|
Age (yr) |
70 (32–84) |
|
Sex (male/female) |
41/30 |
|
Primary disease |
|
|
Pancreatic head cancer |
31 (44) |
|
Distal bile duct cancer |
12 (17) |
|
IPMN/IPMC |
12 (17) |
|
Others |
16 (22) |
|
Operative time (min) |
473 (302–719) |
|
Blood loss (mL) |
310 (25–1,155) |
|
Pancreatic texture |
|
|
Soft |
31 (44) |
|
Hard |
40 (56) |
|
Day of first ambulation (POD) |
1 (1–2) |
|
Day of diet initiation (POD) |
4 (3–4) |
|
Day of drain removal (POD) |
3 (1–25) |
|
Pancreatic fistula (grade B/C) |
4/0 |
|
Delayed gastric emptying |
0 |
|
Postoperative hospital stay (POD) |
8 (6–26) |
|
Readmission within 2 wk after discharge |
5 (7) |
References
- 1. Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 2019;49:190-4. ArticlePubMed
- 2. Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer. 6th ed. Kanehara Shuppan; 2022.Article
- 3. Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. Guidelines for perioperative care for pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) recommendations 2019. World J Surg 2020;44:2056-84. ArticlePubMedPDF
- 4. Xiong J, Szatmary P, Huang W, de la Iglesia-Garcia D, Nunes QM, Xia Q, et al. Enhanced Recovery After Surgery program in patients undergoing pancreaticoduodenectomy: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3497. ArticlePubMedPMC
- 5. Pecorelli N, Nobile S, Partelli S, Cardinali L, Crippa S, Balzano G, et al. Enhanced recovery pathways in pancreatic surgery: state of the art. World J Gastroenterol 2016;22:6456-68. ArticlePubMedPMC
- 6. Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, et al. Effect of an Enhanced Recovery After Surgery protocol in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Clin Nutr 2019;38:174-81. ArticlePubMed
- 7. Hwang DW, Kim HJ, Lee JH, Song KB, Kim MH, Lee SK, et al. Effect of Enhanced Recovery After Surgery program on pancreaticoduodenectomy: a randomized controlled trial. J Hepatobiliary Pancreat Sci 2019;26:360-9. ArticlePubMedPDF
- 8. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. ArticlePubMedPDF
- 9. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-7. ArticlePubMed
- 10. Kaido T, Tamai Y, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017;33:195-8. ArticlePubMed
- 11. Shimura M, Tsuchiya T. Changes in serum zinc levels in patients undergoing gastrointestinal surgery. Jpn J Surg Metab Nutr 2015;49:43-51. Article
- 12. Hammad A, Kaido T, Ogawa K, Fujimoto Y, Tomiyama K, Mori A, et al. Perioperative changes in nutritional parameters and impact of graft size in patients undergoing adult living donor liver transplantation. Liver Transpl 2014;20:1486-96. ArticlePubMed
- 13. Iseki M, Mizuma M, Aoki S, Kawaguchi K, Masuda K, Ishida M, et al. What is the impact of zinc deficiency for pancreatectomies in patients with pancreatic ductal adenocarcinoma? Pancreatology 2022;22:270-6. ArticlePubMed
- 14. Kaido T, Hirose S, Miyachi Y. Short postoperative hospital stay after pancreaticoduodenectomy: what is real minimally invasive surgery? Hepatobiliary Surg Nutr 2021;10:853-6. ArticlePubMedPMC
- 15. Teramura K, Nakamura F, Takeuchi S, Imamura K, Watanabe Y, Tamoto E, et al. Usefulness of ERAS® in pancreaticoduodenectomy. Jpn Soc Clin Surg 2018;79:467-75. Article

 , Yosuke Miyachi
, Yosuke Miyachi , Koichiro Mitsuoka
, Koichiro Mitsuoka , Mariko Sambommatsu
, Mariko Sambommatsu




 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN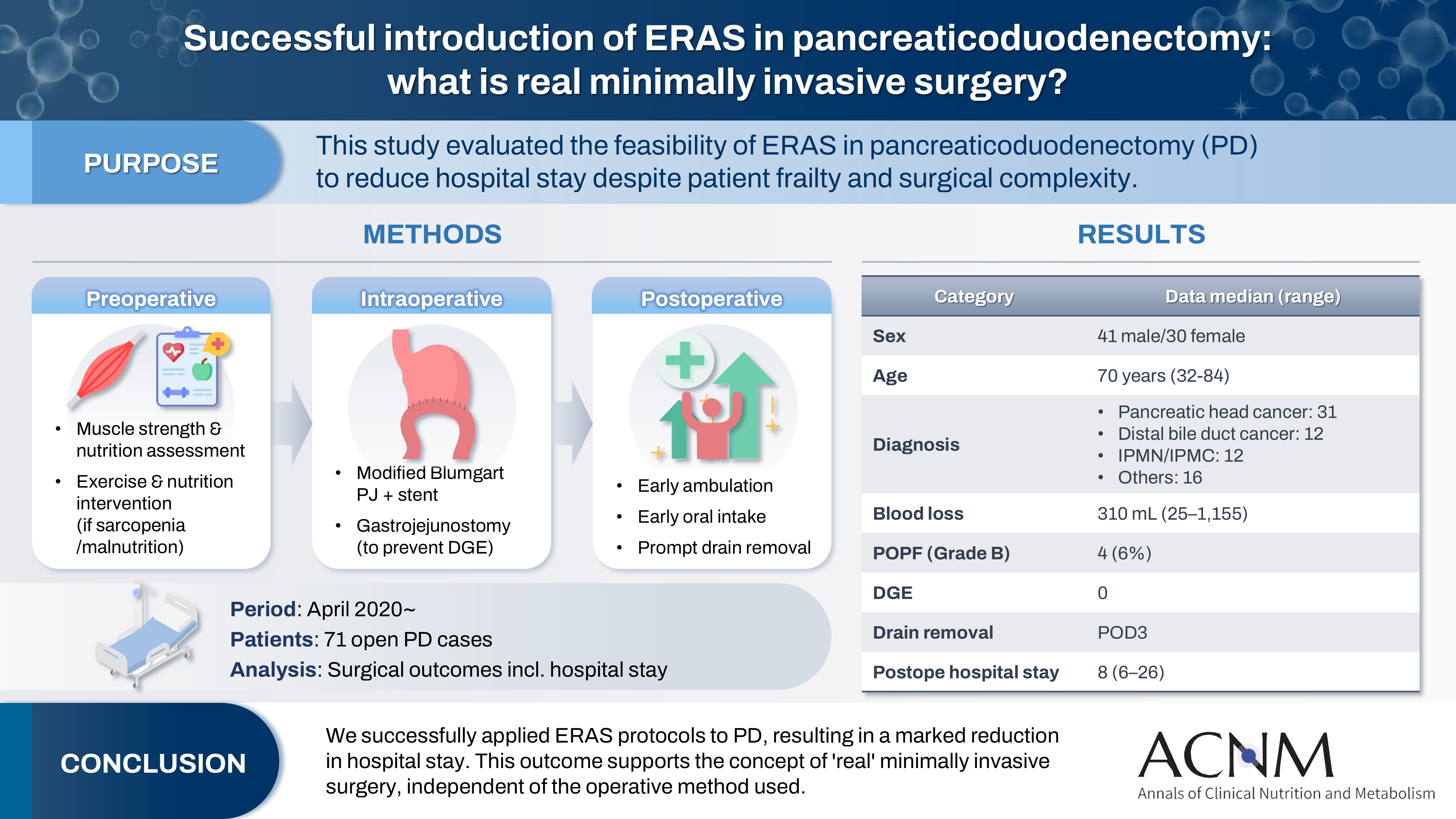
 Cite
Cite

