Indexed in:
Scopus, KCI, KoreaMed
Scopus, KCI, KoreaMed

Search
- Page Path
- HOME > Search
Original Article
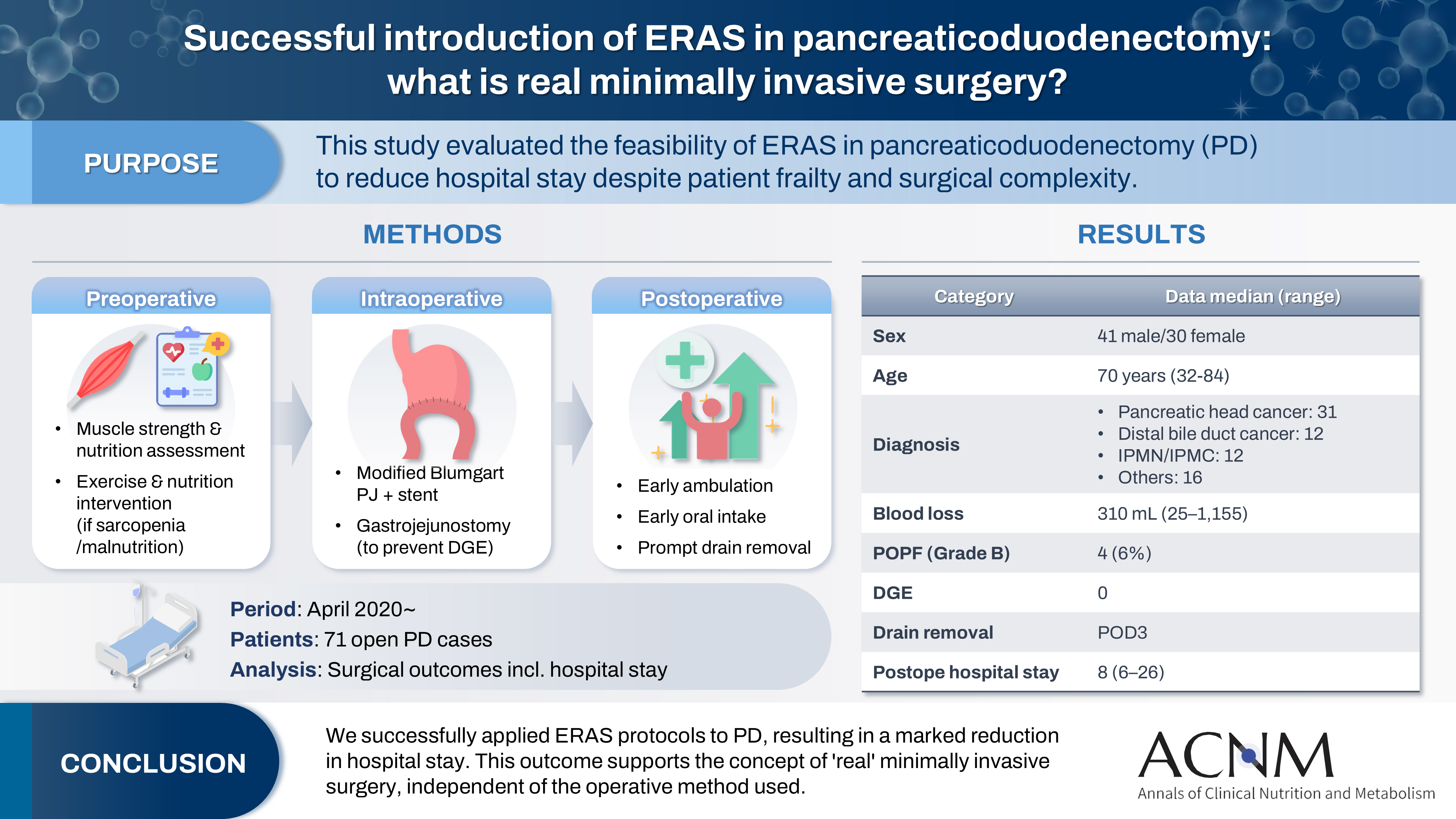
- Successful introduction of ERAS in pancreaticoduodenectomy: what is real minimally invasive surgery?
- Toshimi Kaido, Yosuke Miyachi, Koichiro Mitsuoka, Mariko Sambommatsu
- Ann Clin Nutr Metab 2025;17(2):156-161. Published online August 1, 2025
- DOI: https://doi.org/10.15747/ACNM.25.0014
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Purpose
The introduction of Enhanced Recovery After Surgery (ERAS) protocols for pancreaticoduodenectomy (PD) has been considered challenging due to factors such as preexisting malnutrition, sarcopenia, the complexity of the surgery, and the high incidence of postoperative complications, including postoperative pancreatic fistula (POPF) and delayed gastric emptying (DGE). This study aimed to determine whether ERAS could be implemented in PD to achieve shorter postoperative hospital stays.
Methods
Our novel approach consists of three components. Preoperatively, we routinely assess patients' muscle strength and nutritional status and initiate exercise and nutritional interventions for those identified with sarcopenia or malnutrition. Intraoperatively, we perform pancreaticojejunostomy using a modified Blumgart’s technique with our stent placement policy and utilize new gastrojejunostomy methods to prevent DGE. Principles of postoperative management are early ambulation, early oral intake, and early drain removal. Since April 2020, we have employed this strategy and retrospectively evaluated its effectiveness. We enrolled 71 consecutive patients who underwent open PD with curative intent. Various surgical outcomes, including postoperative hospital stay, were analyzed.
Results
There were 41 men and 30 women, with a median age of 70 years. Preoperative diagnoses included pancreatic head cancer in 31, distal bile duct cancer in 12, and others. Median intraoperative blood loss was 310 mL. Grade B POPF occurred in four patients (6%). No cases of DGE were observed. The median postoperative hospital stay was 8 days (range, 6–26 days).
Conclusion
We successfully implemented ERAS protocols in PD and achieved a significantly reduced postoperative hospital stay. We propose that this approach is “real minimally invasive surgery," regardless of the surgical technique used.
- 3,762 View
- 18 Download

Review
- Perioperative nutritional management to improve long-term outcomes in critically ill perioperative organ transplant patients: a narrative review
- Toshimi Kaido
- Ann Clin Nutr Metab 2025;17(1):18-24. Published online April 1, 2025
- DOI: https://doi.org/10.15747/ACNM.25.0005
-
 Abstract
Abstract
 PDF
PDF - Purpose
This review examines the significance of perioperative nutritional management in organ transplantation, with a particular focus on liver transplantation. Organ transplant recipients often experience malnutrition and sarcopenia due to nutritional and metabolic abnormalities associated with organ dysfunction. Because transplantation is a highly invasive procedure, optimizing perioperative nutritional care is critical for improving short-term outcomes and reducing postoperative infection-related mortality.
Current concept
Recent clinical investigations have shown that liver transplant recipients, who are frequently afflicted with end-stage liver disease and uncompensated cirrhosis, are particularly vulnerable to protein-energy malnutrition and secondary sarcopenia. Our analysis identified low pre-transplant nutritional status and the absence of preoperative branched-chain amino acid supplementation as independent risk factors for post-transplant sepsis. In response, we developed a customized nutritional therapy protocol that incorporates precise body composition analysis, serial measurements of biochemical markers (including prealbumin, zinc, and the branched-chain amino acid/tyrosine ratio), and targeted supplementation with branched-chain amino acids, zinc acetate, and synbiotics. Early initiation of enteral nutrition coupled with postoperative rehabilitative interventions resulted in improved outcomes. In addition, stratified body composition parameters correlated with survival differences and informed revised transplantation criteria.
Conclusion
Tailored perioperative nutritional management and rehabilitative strategies are essential for improving early postoperative outcomes in liver transplantation. These findings underscore the need for proactive nutritional assessment and intervention, which may represent a breakthrough in transplant prognosis. Future research should refine nutritional protocols and integrate novel biomarkers, while education and interdisciplinary collaboration remain crucial for enhancing transplant outcomes and reducing complications. -
Citations
Citations to this article as recorded by- Strengthening collaboration: introducing the contributions of Japanese Society for Surgical Metabolism and Nutrition to Annals of Clinical Nutrition and Metabolism
Ye Rim Chang
Ann Clin Nutr Metab.2025; 17(2): 95. CrossRef
- Strengthening collaboration: introducing the contributions of Japanese Society for Surgical Metabolism and Nutrition to Annals of Clinical Nutrition and Metabolism
- 3,925 View
- 103 Download
- 1 Crossref


 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 First
First Prev
Prev


