Abstract
-
Purpose
Surgical resection is the primary curative treatment for gastrointestinal (GI) cancer; however, it is associated with high postoperative complication rates and impaired recovery. Frailty, malnutrition, and sarcopenia increase morbidity and mortality, underscoring the need for perioperative rehabilitation programs. Standardized rehabilitation protocols during the perioperative period are currently lacking in Korea. We aimed to develop an evidence-based rehabilitation protocol for GI cancer patients to enhance postoperative outcomes and facilitate clinical implementation.
-
Methods
A multidisciplinary task force team comprising experts in surgery, clinical nutrition, and rehabilitation medicine conducted a systematic literature search and comprehensive review from 2012 to 2022 to develop a standardized pre- and re-habilitation protocol for GI cancer surgery. The protocol underwent external validation and subsequent refinements before being finalized through expert consensus.
-
Results
The protocol development process was organized into four consecutive phases: keyword selection, literature review and case report form development, initial protocol drafting, and external validation leading to the final version of the protocol. The final version of the rehabilitation protocol is presented in the main text and included as Supplements.
-
Conclusion
This protocol provides a standardized clinical guideline based on the latest evidence-based pre- and re-habilitation strategies and is designed for seamless integration into routine clinical practice. By facilitating proactive rehabilitation interventions, it aims to improve outcomes in GI cancer patients who are at high risk of postoperative complications, functional decline, and malnutrition.
-
Keywords: Consensus; Frailty; Gastrointestinal tract; Neoplasms; Perioperative period
Introduction
Background
For patients diagnosed with gastrointestinal (GI) cancer, curative surgical resection is generally the only opportunity for long-term survival [
1,
2]. However, it is associated with a high rate of postoperative complications, reported in 40% to 50% of cases [
3-
7]. These complications significantly prolong hospitalization and lead to serious functional impairments [
8-
10]. In particular, patients who develop complications may experience delays or an inability to receive subsequent adjuvant therapy, which negatively impacts long-term oncologic outcomes [
11-
13]. Therefore, identifying risk factors for postoperative complications and implementing proactive interventions to mitigate these risks is crucial. Notably, preoperative frailty and malnutrition have been identified as key predictors of poor outcomes and reduced survival in patients undergoing surgery for GI cancers [
14-
17].
Frailty is characterized by an age-related decline in physical, functional, and cognitive capacities, resulting in impaired physical function and contributing to the development of malnutrition [
18-
20]. Malnutrition is common in GI cancer patients, with a reported prevalence ranging from 20% to 70% [
21]. Similarly, sarcopenia has been reported as an independent risk factor for postoperative complications and poor survival in GI cancers [
22,
23]. Studies have shown that frail patients face a 2- to 4-fold increased risk of postoperative morbidity and mortality [
24-
26]. Given these risks, various interventions aimed at improving preoperative conditions have been explored. The systematic implementation of pre- and re-habilitation programs—including nutritional supplementation and strength training—has been shown to improve quality of life, enhance functional recovery, and reduce major postoperative complications [
27]. Furthermore, the interplay of preexisting frailty or malnutrition, the physiological stress of surgery, and postoperative bed rest with reduced physical activity further impairs physical function and cardiopulmonary capacity, thereby increasing the risk of complications [
28]. Therefore, beyond preoperative interventions, subsequent rehabilitation during postoperative recovery is essential for improving outcomes. Despite this need, no standardized protocols for pre- and re-habilitation in GI cancer surgery currently exist in Korea, and many hospitals continue to rely on individually developed protocols or administer rehabilitation therapy without a formal framework.
In this context, our study aimed to systematically develop a standardized protocol for pre- and re-habilitation therapy for GI cancer surgery. Ideally, rehabilitation therapy should be delivered through the coordinated efforts of a multidisciplinary team involving various healthcare professionals. To ensure that the protocol was comprehensive and widely accepted, we engaged experts from multiple disciplines—surgeons, physiatrists, and dietitians—in the protocol development process [
29]. Our aim is to provide clinical practice guidelines and fundamental resources that will enable healthcare professionals to implement standardized, high-quality pre- and re-habilitation therapy for GI cancer patients, whether they are preparing for surgery or recovering postoperatively.
Methods
Ethics statement
This study was exempt from IRB approval since it does not involve a human population.
Setting
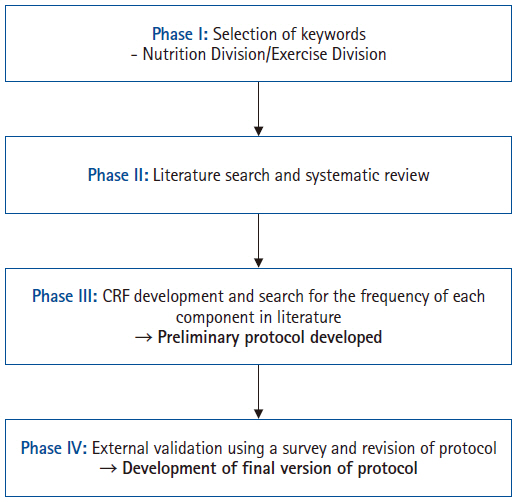
To develop a pre- and re-habilitation protocol for patients undergoing GI cancer surgery, a multidisciplinary task force team (TFT) was established under the leadership of the Korean Society of Surgical Metabolism and Nutrition, with recommendations from the Korean Society of Clinical Nutrition and the Korean Society of Cancer Rehabilitation. The TFT comprised ten board-certified surgeons, five physiatrists, and four clinical dietitians from university hospitals and tertiary referral centers in Korea. The protocol development process was divided into four sequential phases to ensure a systematic and comprehensive approach from 2012 to 2022 (
Fig. 1).
For the development of a comprehensive pre- and re-habilitation protocol, the TFT was subdivided into two specialized subgroups—the Nutrition Division and the Exercise Division—based on each member’s expertise. Each subgroup conducted discussions to identify primary key terms related to nutritional and exercise implementation for rehabilitation therapy. These initial key terms were then presented to all TFT members, and consensus was reached to finalize the key terms for the Phase II literature review.
Phase II
A systematic literature review was conducted using the final set of selected keywords. The search covered articles published between October 2012 and October 2022 in online databases, including PubMed, Web of Science, and the Library of Congress. The inclusion criteria were as follows: studies reporting the effectiveness of rehabilitation therapy for adult cancer patients, articles written in English, full-text availability, and studies categorized as randomized controlled trials or quasi-experimental studies. During the initial abstract screening, articles were excluded if they were in a non-English language, lacked full-text availability, involved a pediatric patient population, employed a non-randomized or non-quasi-experimental design, or were duplicate records. Subsequently, full-text reviews were conducted, and studies were further excluded if their research objectives were not aligned with the purpose of the review or if they did not meet the required study design criteria.
Phase III
In Phase III, the studies selected from the systematic literature review were analyzed and categorized into diagnostic and assessment tools, nutritional rehabilitation, and exercise rehabilitation. Based on these categories, a case report form (CRF) was developed and distributed among TFT members, who conducted a secondary literature review and evaluated the frequency of each item. This process led to the development of the preliminary draft of the pre- and re-habilitation protocol.
Phase IV
To externally validate the preliminary draft of the protocol, feedback was sought from experts affiliated with relevant professional organizations and societies, including the Korean Society of Surgical Metabolism and Nutrition, the Korean Surgical Society, the Korean Society of Cancer Rehabilitation, and the Korean Society of Clinical Nutrition. Additionally, a public hearing was held at the 40th Congress of the KSSMN in October 2024, where feedback and validation were obtained from attending surgeons to ensure the protocol’s clinical relevance and feasibility.
Each question in the preliminary draft of the pre- and re-habilitation protocol was presented with four answer options, allowing respondents to select only one; multiple responses were not permitted. Options were scored as follows: A, strongly agree (4 points); B, somewhat agree (3 points); C, somewhat disagree (2 points); and D, strongly disagree (1 point). The content validity index (CVI) was subsequently calculated for each item using the method proposed by Lynn [
26]. An item was considered to have achieved significant agreement if a consensus rate of 78% or higher was reached, and it was then selected as a valid recommendation. Additionally, the average item-level CVI (I-CVI) was calculated, and if it exceeded 0.8, the entire scale was deemed to have acceptable content validity. Respondents were encouraged to provide additional comments or opposing opinions. If any item required modification, feedback on suggested revisions was collected, and opinions were sought regarding the need for new items and overall comments on the protocol. All feedback was further discussed by TFT members to determine whether to revise or supplement each question.
Based on the survey results, the initial protocol draft was revised, and a final version was developed in accordance with the consensus on the recommendations. Ultimately, a comprehensive pre- and re-habilitation protocol for the perioperative care of GI cancer surgery was created to serve as a practical guideline and to be considered for future updates in clinical practice guidelines.
Results
Phase I
In the Nutrition Division, the first subgroup discussion categorized keywords into four main domains: disease, procedure, nutrition, and outcome. In the disease category, three keywords were selected: cancer, malignancy, and sarcopenia. For the procedure category, five keywords were chosen: surgery, operative, surgical procedures, prehabilitation, and rehabilitation. In the nutrition category, six keywords were selected: nutrition, nutritional assessment, nutritional screening, nutritional intervention, nutritional support, and nutritional therapy. For the outcome category, no specific keywords were included to avoid restricting the search results. The initial set of 14 keywords selected during the first discussion was subsequently refined through a second round of discussions with all TFT members, resulting in a final selection of nine keywords (
Table 1).
In the Exercise Division, the subgroup discussion categorized keywords into three main domains: subject, evaluation, and therapy. During the first discussion, five keywords were selected for the subject category: surgical procedures, operative, digestive system, surgical procedures, and neoplasm. For the evaluation category, seven keywords were chosen: sarcopenia, muscle weakness, frailty, body constitution, walking speed, muscle strength, and physical fitness. In the therapy category, five keywords were selected: preoperative exercise, rehabilitation, physical therapy, exercise, and physical therapy modalities. Out of the 17 keywords identified during the initial discussion, the second round of discussions with all TFT members refined the list, resulting in a final selection of 13 keywords (
Table 1).
A systematic literature review was conducted using the final set of selected keywords. For the Nutrition Division team, the search terms included: (“gastrointestinal” OR “stomach” OR “colorectal” OR “colon” OR “liver” OR “hepatobiliary” OR “pancreas” OR “pancreatobiliary”) AND (“cancer” OR “malignancy”) AND (“surgery” OR “operative” OR “surgical procedures”) AND (“nutrition assessment” OR “nutrition therapy”) AND (“prehabilitation” OR “rehabilitation”). For the Exercise Division team, the search terms included: (“gastrointestinal” OR “stomach” OR “colorectal” OR “colon” OR “liver” OR “hepatobiliary” OR “pancreas” OR “pancreatobiliary”) AND (“cancer” OR “malignancy”) AND (“sarcopenia” OR “muscle weakness” OR “frailty” OR “body composition” OR “walking speed” OR “muscle strength” OR “physical fitness”) AND (“preoperative exercise” OR “physical therapy modalities” OR “exercise”) AND (“rehabilitation”). In total, 93,316 articles were retrieved from the search results, with 71,736 articles related to exercise therapy and 21,580 articles related to nutrition therapy. These articles were distributed among the TFT members for review and screening. After applying the inclusion criteria, a total of 45 articles (22 on nutrition therapy and 23 on exercise therapy) were finally selected for the study.
Phase III
The CRF was developed based on the 45 final selected studies and categorized into six main sections: diagnostic criteria, nutritional assessment tool, muscle status evaluation (including sarcopenia assessment), nutritional intervention, exercise intervention, and outcome assessment tool. For each section, all assessment tools and intervention methods presented in the selected studies were thoroughly reviewed and classified into corresponding subcategories to create a comprehensive CRF. Based on these results, the most frequently occurring items were selected, and the preliminary draft of a pre- and re-habilitation protocol for GI cancer patients undergoing surgery was developed. This draft includes a summary organized into three sections: diagnostic exam and assessment tools; nutritional rehabilitation in the perioperative period; and exercise rehabilitation in the perioperative period. The diagnostic criteria and assessment tools for the protocol were defined as follows. The exclusion criteria for GI cancer patients included illiteracy, dementia, cognitive impairment, inability to perform physical activity or consume oral intake, and cases with distant metastases where surgical resection was not feasible. For nutritional assessment, the Nutrition Risk Screening (NRS) and Patient-Generated Subjective Global Assessment (PG-SGA) were utilized. Nutritional status was classified as malnutrition if the NRS score was ≥3 and the PG-SGA category was B or C. In such cases, nutritional intervention was implemented according to the pre-rehabilitation protocol. In addition to these tools, laboratory tests, body weight changes, and anthropometric measures such as body mass index were also recommended. Nutritional assessments were advised both preoperatively and postoperatively.
For the diagnosis of sarcopenia, body composition analysis was performed and handgrip strength testing was used to assess muscle strength. To evaluate muscle mass, the use of at least one of the following tools was recommended: dual-energy X-ray absorptiometry, bioelectrical impedance analysis, or computed tomography scan. The criteria for sarcopenia diagnosis followed the Asian Sarcopenia Guidelines [
30]. Although surveys such as the 36-Item Short Form (SF-36) and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) were considered for sarcopenia assessment, they were not designated as mandatory components of the protocol.
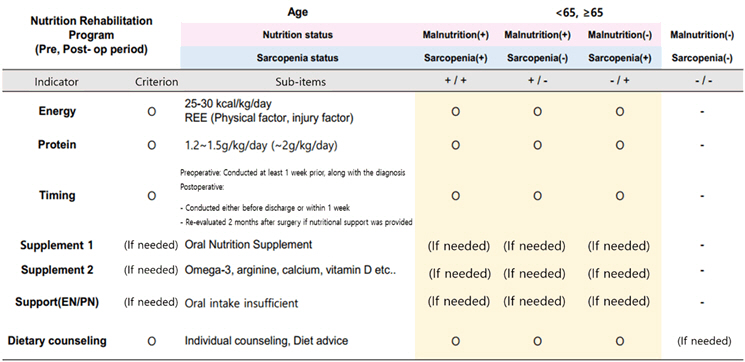
The perioperative nutritional rehabilitation treatment recommends providing 25–30 kcal/kg/day of energy and 1.0–1.5 g/kg/day of protein for adults preparing for surgery, with adjustments made according to the patient’s clinical condition. For patients diagnosed with malnutrition or sarcopenia, the use of oral nutritional supplements (ONS) should be considered both preoperatively and postoperatively, with a recommendation to provide at least 400 kcal/day divided into two or more servings. In cases where oral intake is insufficient, parenteral nutrition supplementation may be considered, along with personalized nutritional management through counseling and education.
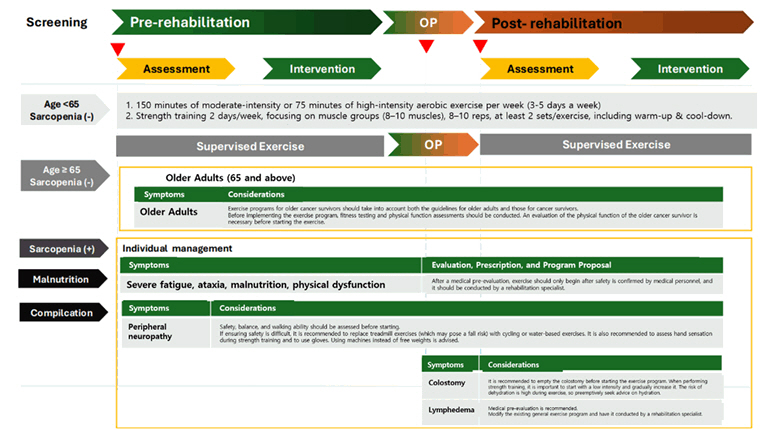
For perioperative exercise rehabilitation treatment, 150 minutes of moderate-intensity or 75 minutes of high-intensity aerobic exercise per week, combined with resistance training twice a week, was recommended. Breathing exercises were encouraged as part of the pre- and re-habilitation program. The type and intensity of exercise should be modified according to the patient’s medical condition. It was emphasized that patients with stomas, lymphedema, severe sarcopenia, frailty, or malnutrition must undergo a safety evaluation by medical professionals before initiating exercise therapy. The frequency analysis results of each CRF item for the development of the preliminary draft of the pre- and re-habilitation protocol are presented in
Supplement 1.
To collect feedback and validate the preliminary draft of the protocol, a questionnaire was developed to assess agreement on each component. The survey was conducted during public hearings and expert meetings to gather additional opinions and gauge consensus. The survey results, including the I-CVI, are presented in
Table 2. Among the 23 items, 21 (91.3%) achieved an I-CVI of 0.78 or higher, indicating that they were considered valid recommendations. The average I-CVI across all items was 0.918, confirming that the overall scale demonstrated an acceptable level of content validity.
During the survey and discussion process, two items (8.7%) received additional comments or opposing opinions from experts. One notable example was item 1-9, which concerned the selection of diagnostic tools for physical function tests in sarcopenia assessment. The recommendation was to use the 6-minute walk test (6MWT) as the primary evaluation tool, with cardiopulmonary exercise testing performed when feasible. However, this item received an I-CVI of 0.61, with 39% of respondents expressing disagreement (18% somewhat disagree and 21% strongly disagree). A counterargument to item 1-9 suggested that, since multiple methods exist for conducting functional tests, the assessment should not be limited solely to the 6MWT. Additionally, some institutions noted practical limitations in performing tests such as the 6MWT. Based on TFT discussions, it was determined that both the Short Physical Performance Battery—which includes the standing balance test, gait velocity test, and repeated chair stands—and the 6MWT should be included as diagnostic tools for physical function assessment in sarcopenia evaluation of GI cancer patients. When feasible, cardiopulmonary exercise testing was recommended as a supplementary assessment to further evaluate cardiopulmonary capacity and functional status.
Regarding item 2-3, which recommended that the determination and evaluation of insufficient oral intake be left to the clinical judgment of healthcare professionals or clinical dietitians, the I-CVI was 0.68, with 32% of respondents expressing disagreement (19% somewhat disagree and 13% strongly disagree). A comment suggested changing the phrase “based on the judgment of medical staff or clinical dietitians” to “based on the judgment of medical staff, including clinical dietitians,” since clinical dietitians are also part of the medical staff. Consequently, the wording of this item was revised according to the suggested feedback. Additionally, some respondents raised concerns about whether all clinical dietitians could reliably perform the PG-SGA assessment (item 1-2, I-CVI 0.87, with four respondents expressing some or strong disagreement). However, consensus was reached that this would not pose a significant issue. For item 1-6 concerning the timing of nutritional pre- and re-habilitation interventions, some concerns were raised that “if necessary, preoperative nutritional therapy should last at least 2 weeks, so starting it only 1 week prior is too late” (I-CVI 0.91, with three respondents somewhat disagreeing). Nevertheless, it was agreed that setting a minimum standard based on realistic institutional circumstances was more appropriate, and the current recommendation was maintained.
After the revision process based on expert discussions and survey results, all modified items were reviewed until complete consensus was reached among TFT members. The final version of the pre- and re-habilitation protocol for GI cancer surgery was then developed (
Appendices 1,
2,
Supplements 2).
Discussion
We have systematically developed a structured pre- and re-habilitation protocol for patients undergoing GI tract cancer surgery to ensure effective implementation in clinical practice. This process involved a comprehensive review of international guidelines and the latest evidence from the literature. To enhance its clinical applicability, the protocol was designed to be highly specific and sequentially structured, covering key aspects such as patient assessment, nutritional and exercise therapy, and outcome evaluation. It was meticulously formulated to enable immediate use in clinical settings. Furthermore, the protocol underwent a rigorous review and approval process by a multidisciplinary team of experts and healthcare professionals to facilitate its adoption into routine practice. To our knowledge, this is the first standardized protocol systematically compiled to guide clinical decision-making in the pre- and re-habilitation management of GI tract cancer surgery in Korea.
Most cancer patients experience not only physical and functional decline but also systemic inflammatory changes and frailty. Additionally, individuals aged 65 years or older constitute the majority of cancer patients undergoing surgery [
31]. Among these patients, approximately 40% are reported to be frail, and nearly 20% of frail older adults develop new postoperative disabilities that impair their ability to perform daily activities [
32]. Frailty is characterized by a decline in physiological and physical reserves, leading to reduced adaptability to external stressors and increased vulnerability to disease [
33-
35]. It is also associated with more than a twofold increase in postoperative complication rates, mortality, and the likelihood of discharge to long-term care facilities compared to non-frail patients. With the acceleration of population aging, the number of frail older adults undergoing surgery is expected to rise, further increasing the risk of postoperative adverse events. Pre- and re-habilitation aims to enhance physiological and functional reserves before surgery, thereby mitigating postoperative functional decline and facilitating a faster return to preoperative performance levels. The role of rehabilitation in improving surgical outcomes for frail patients is increasingly emphasized, as it has been recognized as a key strategy for reducing postoperative complications and promoting functional recovery. Therefore, the standardized protocol developed in this study should be regarded as an essential therapeutic intervention rather than merely an adjunctive treatment. It is intended for active implementation in patients who are particularly vulnerable to physiological changes and surgical stress, and it is expected that postoperative outcomes and long-term prognoses will improve in high-risk populations, while also supporting preoperative functional recovery. However, some previously published studies on frail older adults have failed to demonstrate significant improvements in postoperative function or complication rates following perioperative rehabilitation, often reporting an average protocol adherence rate below 60%. In contrast, when analyses were restricted to participants who completed at least 80% of the rehabilitation protocol, significant reductions in complication rates and improved recovery of pre-rehabilitation functional levels were observed [
36]. Therefore, to confirm the clinical efficacy of pre- and re-habilitation protocols, efforts should be made to maintain an optimal adherence rate of at least 80%. This goal necessitates continuous investigation and intervention to identify and address factors that may hinder protocol adherence, ultimately ensuring the effective implementation of these programs.
This protocol was developed based on the latest research findings and international guidelines. However, certain details remain subject to ongoing discussion, as some principles are not yet firmly established or may require individualized application depending on specific clinical conditions. Therefore, ongoing refinement through further research and revisions may be necessary. For example, the recommended energy intake (25–30 kcal/kg/day) and protein intake (1.0–1.5 g/kg/day) in items 1-1 and 1-2 of the Nutritional Rehabilitation Program were determined based on the American Society for Parenteral and Enteral Nutrition [
37] and European Society for Clinical Nutrition and Metabolism guidelines [
38], with approval from the committee and external experts. Nonetheless, in patients with specific risk factors such as advanced age or sarcopenia, as well as those with underlying conditions like renal or hepatic failure, individualized targets should be considered. Given that this protocol primarily aims to establish standardized clinical guidelines for pre- and re-habilitation in GI tract cancer surgery, it does not include detailed specifications for every individual parameter.
Enteral immunonutrition using ONS containing omega-3 fatty acids, glutamine, arginine, and nucleotides has recently attracted growing interest for perioperative nutritional support in cancer patients. However, despite its theoretical advantages, meta-analyses have produced inconclusive results. The heterogeneity of the studies—resulting from variations in cancer types, surgical factors, and individual nutritional statuses—has made it difficult to draw definitive conclusions. As a result, major nutritional guidelines present varying recommendations for preoperative ONS and immunonutrition. For example, the ASPEN guidelines recommend that preoperative ONS for 5–7 days may benefit malnourished patients prior to surgery [
37]. In contrast, the ESPEN guidelines endorse preoperative ONS but highlight the lack of clear evidence favoring immunomodulating ONS formulations (e.g., those containing arginine, omega-3 fatty acids, and nucleotides) over standard ONS [
38]. Although immunonutrition may be considered, the guidelines do not offer a standardized recommendation due to insufficient evidence. In our protocol, perioperative ONS use (items 2-5 to 2-7) is recommended, with a minimum daily intake of 400 kcal split into at least two doses. The use of immunomodulating ONS containing omega-3 fatty acids and arginine is suggested only when deemed necessary. Future updates and revisions of this protocol should include more detailed specifications of certain nutritional therapy items to enhance clinical applicability.
The exercise program outlined in this protocol recommends high-intensity aerobic and resistance exercises for all cancer patients who are capable of participating in pre- and re-habilitation, regardless of age, while also incorporating respiratory rehabilitation to account for surgical characteristics (item 3-1). Resistance exercise directly stimulates protein synthesis in skeletal muscle, with synthesis rates increasing in proportion to exercise intensity. Aerobic exercise training improves maximal oxygen uptake, mitochondrial oxidative enzyme activity, and insulin sensitivity. When combined with resistance exercise, it can further enhance protein synthesis. Moreover, regular physical exercise induces anti-inflammatory cytokines and helps mitigate muscle wasting associated with cancer-related inflammation, benefiting patients recovering from major surgery who often exhibit systemic inflammatory responses postoperatively. Despite these advantages, concerns may arise regarding the safety of implementing exercise pre- and re-habilitation for high-risk cancer patients, such as those who are elderly or malnourished. However, recent studies support the feasibility and safety of exercise even in high-risk older patients. For example, Chia et al. [
39] conducted a perioperative exercise rehabilitation program in colorectal cancer patients with a mean age of 79 years who had preoperative frailty and reported an adherence rate exceeding 80%. Similarly, Karlsson et al. [
40] investigated preoperative exercise rehabilitation in colorectal cancer patients with a mean age of 83.5 years, observing a compliance rate of 97% with no critical complications. These findings suggest that, when tailored appropriately in terms of intensity and frequency to each patient’s characteristics, exercise pre- and re-habilitation—including both resistance and aerobic components—can be safely implemented in elderly, high-risk patients. Therefore, as highlighted in items 3-2 and 3-3, individualized exercise programs should be administered under the supervision of rehabilitation specialists within a multidisciplinary team. Further refinements should be made to specify detailed considerations based on patients’ underlying conditions and frailty levels.
This protocol has some limitations. It was developed with the primary objective of establishing comprehensive guidelines for a diverse population of patients with GI cancer. However, due to the limited number of well-established randomized controlled trials specifically targeting GI surgery patients, this protocol was formulated based on a systematic literature review, integrating the frequency of individual components and expert consensus. This approach may represent a methodological limitation. Furthermore, this study did not create cancer type–specific protocols or differentiate detailed subcategories according to patients’ comorbidities, disease staging, or clinical conditions. Future research should therefore focus on developing more specialized and stratified sub-protocols that account for variations in cancer types, comorbidities, and pathological characteristics. Additionally, this protocol does not offer a framework for evaluating the feasibility and effectiveness of implementing pre- and re-habilitation strategies in clinical practice. Objective assessment of clinical outcomes and guideline adherence is essential to ensure successful protocol execution and to optimize its impact. Consequently, future studies should establish standardized criteria and assessment tools to measure both the appropriateness of implementation and the clinical efficacy of each component of the protocol.
Conclusion
Upon completion, the final version of this protocol received official endorsement from the Korean Society of Surgical Metabolism and Nutrition. To promote its adoption in clinical practice, the protocol will be disseminated via email and official websites to medical professionals affiliated with relevant academic societies, including the Korean Society of Surgical Metabolism and Nutrition, the Korean Surgical Society, the Korean Society of Cancer Rehabilitation, and the Korean Society of Clinical Nutrition. By providing the latest evidence on perioperative rehabilitation and a standardized clinical guideline that can be easily integrated into routine practice, this protocol is expected to facilitate proactive pre- and re-habilitation interventions for GI tract cancer patients at high risk of postoperative complications, functional decline, and malnutrition. Consequently, it may contribute to improved postoperative outcomes and better long-term prognoses for these patients.
Appendix
- Appendix 1. The final version of the pre- and re-habilitation protocol for gastrointestinal cancer patients.
I. Diagnostic Exam and Assessment Tool for Pre- and Postoperative Rehabilitation
1-1. Exclusion Criteria
The following patients should be excluded from this prehabilitation protocol if they meet any of the criteria below.
A. Illiteracy/Dementia/Cognitive impairment
B. Inability to ambulate or perform physical activity
C. Inability to consume food orally
D. Presence of distant metastases unsuitable for surgical resection
1-2. Nutrition Screening & Assessment
A. Nutrition screening should be performed using NRS (Nutritional Risk Screening), and patients scoring 3 or higher are recommended to refer to a clinical dietitian.
B. The clinical dietitian evaluates nutritional status using the PG-SGA (Patient-Generated Subjective Global Assessment) and considers nutritional intervention.
C. Based on nutritional status:
• If the patient has adequate nutrition (NRS ≥3, PG-SGA A), they are recommended to receive Standard Care.
• If the patient is malnourished (NRS ≥3, PG-SGA B or C), they are recommended to follow the Nutrition Intervention Care under the pre-rehabilitation protocol.
1-3. Nutritional Assessment (Food Intake Record)
A. Pre- and postoperatively, a 3-day food record or 24-hour dietary recall can be used to evaluate the patient’s usual intake to assess the effects of nutritional intervention and set management goals.
B. However, this is optional and may be omitted if necessary.
1-4. Nutritional Assessment (Laboratory Tool)
A. Pre- and postoperative blood tests should be performed, including albumin, pre-albumin, hemoglobin, and C-reactive protein.
B. If feasible, measure TLC (total lymphocyte count), transferrin, and vitamin D as well.
1-5. Nutritional Assessment (Anthropometric)
A. Pre- and postoperatively, assess weight changes and BMI (body mass index) as indicators of nutritional status.
B. Body composition analysis for sarcopenia diagnosis is essential. If possible, assess fat mass (FM) and lean body mass (LBM).
1-6. Nutritional Assessment (Timing)
A. Nutritional assessments should be performed both pre- and postoperatively.
B. It is recommended that preoperative assessments be conducted at least one week before surgery along with the diagnosis.
C. It is recommended that postoperative assessments be done before discharge or on the 7th postoperative day. For patients who underwent nutritional intervention due to malnutrition, reassessment should be conducted 2 months after surgery.
1-7. Sarcopenia Evaluation (Muscle Strength Test)
A. Muscle strength for sarcopenia evaluation is recommended to be assessed using the Handgrip Strength Test.
B. Measure three times and calculate the average value for evaluation. C. Cutoff values: male <28 kg, female <18 kg (Asian Sarcopenia Guidelines).
1-8. Sarcopenia Evaluation (Muscle Mass)
A. Muscle mass for sarcopenia evaluation is recommended to be measured using DEXA, BIA, or CT scans.
B. Cutoff values (Asian Sarcopenia Guidelines):
• DEXA: SMI; Male <7.0 kg/m², Female <5.4 kg/m²
• BIA: Male <7.0 kg/m², Female <5.7 kg/m²
1-9. Sarcopenia Evaluation (Physical Function Test)
A. Physical function for sarcopenia evaluation is recommended to be assessed using both the Short Physical Performance Battery (SPPB)—comprising the standing balance test, gait velocity test, and repeated chair stands—and the 6-Minute Walk Test.
B. CPET (Cardiopulmonary Exercise Test) may be performed if available, as it provides comprehensive evaluation of exercise capacity and cardiopulmonary function.
1-10. Sarcopenia Evaluation (Questionnaire)
A. Quality of life assessment for sarcopenia evaluation can consider using SF-36 or EORTC QLQ-C30, but it is not mandatory.
II. Nutritional Rehabilitation Program in Pre- and Postoperative Period
2-1. Energy requirements should be determined considering the patient’s physical condition, metabolic status, and disease impact. For adults undergoing surgery, 25–30 kcal/kg/day of energy is recommended. For elderly patients, providing more than 30 kcal/kg/day is recommended. However, this requirement may be adjusted according to the patient’s condition.
2-2. Protein is essential for maintaining and restoring muscle mass after surgery. Generally, 1.0–1.5 g/kg/day of protein is recommended. For elderly patients or those at risk of sarcopenia, at least 1.2 g/kg/day of protein should be provided.
Answer: A. Strongly Agree B. Somewhat Agree C. Somewhat Disagree D. Strongly Disagree
2-3. The evaluation of insufficient oral intake should be based on the judgment of medical staff, including clinical dietitians.
2-4. If malnutrition or sarcopenia is present, the use of oral nutritional supplements (ONS) should be considered in the pre- and postoperative periods.
2-5. Oral nutritional supplements (ONS) should provide at least 400 kcal/day.
2-6. Oral nutritional supplements (ONS) should be administered in two or more divided doses per day.
2-7. If necessary, select oral nutritional supplements containing Omega-3 or Arginine.
2-8. In cases of insufficient oral intake in the pre- and postoperative periods, parenteral nutrition (PN) should be applied as needed.
2-9. If necessary, refer to a clinical dietitian for personalized nutritional counseling and education for tailored nutrition management
III. Exercise Rehabilitation Program in Pre- and Postoperative Period
3-1. For healthy cancer patients, it is recommended that the exercise prescription include 150 minutes of moderate-intensity aerobic exercise or 75 minutes of high-intensity aerobic exercise per week (3–5 days per week), along with resistance exercises twice a week involving 8–10 muscle groups with 8–10 repetitions for at least 2 sets. Breathing exercises are also recommended to reduce postoperative complications.
3-2. Patients with a low risk of exercise-related complications may transition from a hospital-based exercise program to a home-based exercise program. However, for patients with a high risk of exercise-related complications, a supervised exercise program is required.
3-3. For elderly patients or those with sarcopenia, a comprehensive assessment of the patient’s medical condition should be conducted, and a structured exercise program tailored to the individual’s physical status—including the type and intensity of exercises—should be provided.
3-4. For patients with comorbidities, stomas, lymphedema, severe sarcopenia (frailty), or severe malnutrition, a medical pre-evaluation must be conducted. Exercise should only be initiated after medical safety confirmation by healthcare providers and performed under the supervision of rehabilitation specialists.
- Appendix 2. Summary of the pre- and re-habilitation protocol for gastrointestinal cancer patients.
I. Enrollment Criteria and Assessment of Malnutrition and Sarcopenia
1. Inclusion and Exclusion
1. This protocol targets patients diagnosed with gastrointestinal cancer (stomach cancer, colon cancer, liver cancer, bile duct cancer, and pancreatic cancer) who have undergone surgery.
2. Patients who meet any of the following criteria will be excluded:
• Illiterate / Dementia / Cognitive impairment
• Unable to move / Unable to exercise
• Unable to intake food
• Presence of distant metastasis that cannot be surgically resected
2. Malnutrition Assessment
1. Nutrition Risk Screening (NRS) is used as a nutrition screening tool, and if the score is 3 or higher, the patient is referred to a clinical nutritionist.
2. The clinical nutritionist assesses the nutritional status using Patient-Generated Subjective Global Assessment (PG-SGA) and considers nutritional interventions.
3. Depending on the nutritional status, if the patient is in good nutritional condition (NRS<3, PG-SGA A), standard care will be implemented. If the patient is in a malnourished state (NRS≥3, PG-SGA B or C), the pre-rehabilitation protocol under Nutrition Intervention Care will be followed.
4. Dietary intake records can be made using a 3-day food diary or a 24-hour recall method, though it is not mandatory.
5. Pre- and post-surgery, blood tests (albumin, pre-albumin, hemoglobin, CRP) are performed.
6. If possible, TLC, transferrin, and vitamin D should also be measured.
7. Pre- and post-surgery, weight changes and body mass index (BMI) are assessed as indicators of nutritional status.
8. Nutritional assessment is performed both before and after surgery.
9. The pre-surgery assessment should be conducted at least 1 week before surgery, along with the diagnosis.
10. Post-surgery, the assessment is conducted either before discharge or within 1 week. For patients who underwent intervention due to malnutrition, the assessment is done 2 months after surgery.
3. Sarcopenia Assessment
1. The muscle strength evaluation for sarcopenia assessment is conducted using the handgrip test.
• A total of 3 measurements are taken, and the average value is calculated for assessment.
• Reference values: Men <28 kg, Women <18 kg (Asian Sarcopenia Guideline)
2. The muscle mass evaluation for sarcopenia assessment is conducted using one of the following methods: DEXA, BIA, or CT scan. • DEXA: SMI; Men <7.0 kg/m², Women <5.4 kg/m² • BIA: SMI; Men <7.0 kg/m², Women <5.7 kg/m²
3. The physical function evaluation for sarcopenia assessment is conducted using the 6-minute walk test or SPPB.
4. CPET, which allows comprehensive evaluation of exercise capacity and cardiopulmonary function, should be performed if possible.
5. As a quality of life-related survey for sarcopenia assessment, SF-36 and EORTC QLQ-C30 may be considered, but they are not mandatory.
II. Summary of Nutrition Pre- and Re-habilitation: Assessment and Intervention
1. Nutrition assessment
2. Nutrition intervention
III. Summary of Exercise Pre- and Re-habilitation: Assessment and Intervention
Authors’ contribution
Conceptualization: IKL, EYK. Methodology: IKL, EYK, JHB. Formal analysis/validation: JK, EJY, GYK, DHK, JAK, JSK, KEN, SUB, JHP, SYA, EYK, JHB. Project administration: SJP, SYO, SJY, SHJ, NJC, JHH. Funding acquisition: IKL. Writing – original draft: EYK. Writing – review & editing: EYK, IKL. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that there are no potential conflicts of interest.
Funding
This study was selected as a policy project and supported by the Korean Society of Surgical Metabolism and Nutrition Research Grant (No. 2024-04).
Data availability
The research data, including the case report form, are available on request from the corresponding author.
Acknowledgements
We would like to express our sincere gratitude to all members of the task force team: Gyeongran Kang (Kyung Hee University Medical Center); Dong Hwan Kim (Department of Physical Medicine and Rehabilitation, Kyung Hee University Hospital at Gangdong, Kyung Hee University College of Medicine, Korea); Jeonga Kim (Department of Clinical Nutrition, Asan Medical Center); Jin Soo Kim (Department of Surgery, Chungnam National University School of Medicine, Korea); Kyung Eun Nam (Department of Rehabilitation Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea); Sung Uk Bae (Department of Surgery, School of Medicine, Dongsan Medical Center, Keimyung University, Korea); Ji-Hyeon Park (Department of Surgery, Gachon University College of Medicine, Gachon University Gil Medical Center, Korea); So Young Ahn (Department of Rehabilitation Medicine, College of Medicine, Chungnam National University, Korea); Seung-Young Oh (Department of Critical Care Medicine, Seoul National University Hospital, Korea); So Jeong Yoon (Division of Hepato-Biliary-Pancreas Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea); Huisong Lee (Department of Surgery, Ewha Womans University, Mokdong Hospital, Korea); Sehwa Joo (Department of Clinical Nutrition, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea); Nak Jun Choi (Division of Acute Care Surgery, Department of Surgery, Korea University Guro Hospital, Korea); and Ji Hye Hwang (Department of Physical and Rehabilitation Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea) for their dedication and hard work on this study. This achievement would not have been possible without their invaluable cooperation.
Supplementary materials
Supplement 1.
The frequency analysis results of each case report form item for the development of the preliminary draft of the pre- and re-habilitation protocol.
Supplement 2.
Summary of the pre- and re-habilitation protocol for gastrointestinal cancer patients. (Korean version)
Fig. 1.The protocol development process. CRF, case report form.
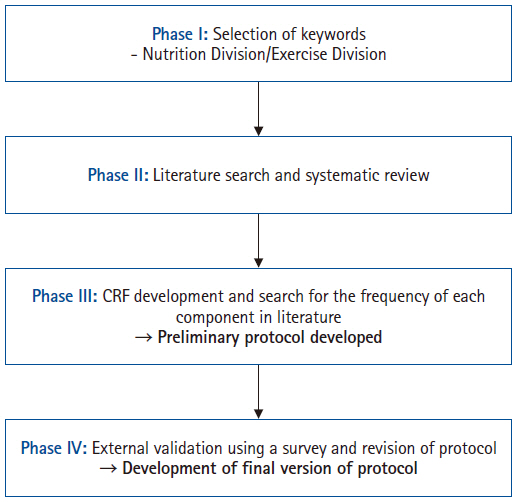
Table 1.Selected keywords for literature review and protocol development
|
Division |
Category |
Initially selected keywords |
Finally selected keywords |
|
Nutrition Division |
· Disease |
Cancer, malignancy, and sarcopenia |
Cancer |
|
Malignancy |
|
Surgery |
|
Operative |
|
Surgical procedures |
|
Nutrition assessment |
|
· Procedure |
Surgery, operative, surgical procedures, prehabilitation, and rehabilitation |
Nutrition therapy |
|
· Nutrition |
Nutrition, nutritional assessment, nutritional screening, nutritional intervention, nutritional support, and nutritional therapy |
Prehabilitation |
|
· Outcome |
-a
|
Rehabilitation |
|
Exercise Division |
· Subject |
Surgical procedures, operative, digestive system, surgical procedures, and neoplasm |
Cancer |
|
Malignancy |
|
Sarcopenia |
|
Muscle weakness |
|
Frailty |
|
Body composition |
|
Walking speed |
|
Muscle strength |
|
Physical fitness |
|
Preoperative exercise |
|
Physical therapy modalities |
|
· Evaluation |
Sarcopenia, muscle weakness, frailty, body constitution, walking speed, muscle strength, and physical fitness |
Exercise |
|
· Therapy |
Preoperative exercise, rehabilitation, physical therapy, exercise, and physical therapy modalities |
Rehabilitation |
Table 2.Results of the questionnaire for validation of the pre- and re-habilitation protocol in gastrointestinal cancer patients
|
Item of protocol |
Score, No. (%) |
Total score |
No. of total respondents |
I-CVIb
|
|
4a
|
3a |
2a |
1a |
|
I. Diagnostic exam and assessment tool for pre- and postoperative rehabilitation |
1-1 |
19 (59) |
8 (25) |
5 (16) |
0 |
110 |
32 |
0.84 |
|
1-2 |
13 (42) |
14 (45) |
3 (10) |
1 (3) |
101 |
31 |
0.87 |
|
1-3 |
18 (56) |
10 (31) |
4 (13) |
0 |
110 |
32 |
0.87 |
|
1-4 |
21 (66) |
10 (31) |
1 (3) |
0 |
116 |
32 |
0.97 |
|
1-5 |
29 (91) |
2 (6) |
1 (3) |
0 |
124 |
32 |
0.97 |
|
1-6 |
7 (21) |
23 (70) |
3 (9) |
0 |
103 |
33 |
0.91 |
|
1-7 |
23 (72) |
4 (12) |
5 (16) |
0 |
114 |
32 |
0.84 |
|
1-8 |
25 (78) |
4 (13) |
2 (6) |
1 (3) |
117 |
32 |
0.91 |
|
1-9 |
3 (9) |
17 (52) |
6 (18) |
7 (21) |
82 |
33 |
0.61 |
|
1-10 |
14 (42) |
17 (52) |
2 (6) |
0 |
111 |
33 |
0.94 |
|
II. Nutritional rehabilitation program in pre- and postoperative period |
2-1 |
20 (69) |
9 (31) |
0 |
0 |
107 |
29 |
1.00 |
|
2-2 |
22 (71) |
8 (26) |
1 (3) |
0 |
114 |
31 |
0.97 |
|
2-3 |
7 (23) |
14 (45) |
6 (19) |
4 (13) |
86 |
31 |
0.68 |
|
2-4 |
21 (68) |
9 (29) |
1 (3) |
0 |
113 |
31 |
0.97 |
|
2-5 |
12 (39) |
18 (58) |
1 (3) |
0 |
104 |
31 |
0.97 |
|
2-6 |
16 (52) |
13 (42) |
2 (6) |
0 |
107 |
31 |
0.94 |
|
2-7 |
18 (58) |
12 (39) |
0 |
1 (3) |
109 |
31 |
0.97 |
|
2-8 |
27 (87) |
3 (10) |
0 |
1 (3) |
118 |
31 |
0.97 |
|
2-9 |
24 (78) |
5 (16) |
2 (6) |
0 |
115 |
31 |
0.94 |
|
III. Exercise rehabilitation program in pre- and postoperative period |
3-1 |
8 (30) |
18 (66) |
1 (4) |
0 |
88 |
27 |
0.96 |
|
3-2 |
24 (89) |
3 (11) |
0 |
0 |
105 |
27 |
1.00 |
|
3-3 |
24 (89) |
3 (11) |
0 |
0 |
105 |
27 |
1.00 |
|
3-4 |
21 (78) |
6 (22) |
0 |
0 |
102 |
27 |
1.00 |
References
- 1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335-49. ArticlePubMedPMC
- 2. Lu L, Mullins CS, Schafmayer C, Zeibig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond) 2021;41:1137-51. ArticlePubMedPMCPDF
- 3. Steffens D, Solomon MJ, Young JM, Koh C, Venchiarutti RL, Lee P, et al. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open 2018;2:328-35. ArticlePubMedPMCPDF
- 4. Venchiarutti RL, Solomon MJ, Koh CE, Young JM, Steffens D. Pushing the boundaries of pelvic exenteration by maintaining survival at the cost of morbidity. Br J Surg 2019;106:1393-403. ArticlePubMedPDF
- 5. Marusawa H, Jenkins BJ. Inflammation and gastrointestinal cancer: an overview. Cancer Lett 2014;345:153-6. ArticlePubMed
- 6. Hii MW, Smithers BM, Gotley DC, Thomas JM, Thomson I, Martin I, et al. Impact of postoperative morbidity on long-term survival after oesophagectomy. Br J Surg 2013;100:95-104. ArticlePubMedPDF
- 7. Collins A, Hatzaras I, Schmidt C, Carruthers K, Melvin WS, Muscarella P, et al. Gastrectomy in advanced gastric cancer effectively palliates symptoms and may improve survival in select patients. J Gastrointest Surg 2014;18:491-6. ArticlePubMedPDF
- 8. Steffens D, Koh C, Ansari N, Solomon MJ, Brown K, McBride K, et al. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: early results from a prospective cohort study of 115 patients. Ann Surg Oncol 2020;27:3986-94. ArticlePubMedPDF
- 9. McBride KE, Steffens D, Solomon MJ, Koh C, Ansari N, Young CJ, et al. Cost-analysis of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal malignancy: an Australian perspective with global application. Eur J Surg Oncol 2021;47:828-33. ArticlePubMed
- 10. Koh CE, Badgery-Parker T, Salkeld G, Young JM, Heriot AG, Solomon MJ. Cost-effectiveness of pelvic exenteration for locally advanced malignancy. Br J Surg 2016;103:1548-56. ArticlePubMedPDF
- 11. Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 2014;21:891-8. ArticlePubMedPDF
- 12. Aurello P, Cinquepalmi M, Petrucciani N, Moschetta G, Antolino L, Felli F, et al. Impact of anastomotic leakage on overall and disease-free survival after surgery for gastric carcinoma: a systematic review. Anticancer Res 2020;40:619-24. ArticlePubMed
- 13. Okumura Y, Hiki N, Kumagai K, Ida S, Nunobe S, Ohashi M, et al. Postoperative prolonged inflammatory response as a poor prognostic factor after curative resection for gastric cancer. World J Surg 2017;41:2611-8. ArticlePubMedPDF
- 14. Steffens D, Young JM, Solomon M, Beckenkamp PR, Koh C, Vuong K, et al. Preliminary evidence for physical activity following pelvic exenteration: a pilot longitudinal cohort study. BMC Cancer 2019;19:661.ArticlePubMedPMCPDF
- 15. Cornet M, Lim C, Salloum C, Lazzati A, Compagnon P, Pascal G, et al. Prognostic value of sarcopenia in liver surgery. J Visc Surg 2015;152:297-304. ArticlePubMed
- 16. Older P, Smith R, Hall A, French C. Preoperative cardiopulmonary risk assessment by cardiopulmonary exercise testing. Crit Care Resusc 2000;2:198-208. ArticlePubMed
- 17. Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013;206:544-50. ArticlePubMedPMC
- 18. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011;27:1-15. ArticlePubMedPMC
- 19. Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med 2011;27:101-10. ArticlePubMedPMC
- 20. Lee L, Heckman G, Molnar FJ. Frailty: identifying elderly patients at high risk of poor outcomes. Can Fam Physician 2015;61:227-31. PubMedPMC
- 21. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. ArticlePubMed
- 22. Kabashneh S, Alkassis S, Shanah L, Ali H. A complete guide to identify and manage malnutrition in hospitalized patients. Cureus 2020;12:e8486.ArticlePubMedPMC
- 23. Saunders J, Smith T. Malnutrition: causes and consequences. Clin Med (Lond) 2010;10:624-7. ArticlePubMedPMC
- 24. van Vugt JL, Levolger S, Coelen RJ, de Bruin RW, IJzermans JN. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol 2015;112:681-2. ArticlePubMedPDF
- 25. Panayi AC, Orkaby AR, Sakthivel D, Endo Y, Varon D, Roh D, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg 2019;218:393-400. ArticlePubMedPMC
- 26. Lynn MR. Determination and quantification of content validity. Nurs Res 1986;35:382-5. ArticlePubMed
- 27. Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50-6. ArticlePubMed
- 28. Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006;91:4836-41. ArticlePubMed
- 29. Kampling H, Reese C, Kust J, Mittag O. Systematic development of practice guidelines for psychological interventions in stroke rehabilitation. Disabil Rehabil 2020;42:1616-22. ArticlePubMed
- 30. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300-7. ArticlePubMed
- 31. Fowler AJ, Abbott TEF, Prowle J, Pearse RM. Age of patients undergoing surgery. Br J Surg 2019;106:1012-8. ArticlePubMedPDF
- 32. McIsaac DI, Taljaard M, Bryson GL, Beaule PE, Gagne S, Hamilton G, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg 2020;271:283-9. ArticlePubMed
- 33. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. ArticlePubMedPMC
- 34. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. ArticlePubMed
- 35. Milder DA, Pillinger NL, Kam PC. The role of prehabilitation in frail surgical patients: a systematic review. Acta Anaesthesiol Scand 2018;62:1356-66. ArticlePubMedPDF
- 36. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 2020;155:233-42. ArticlePubMedPMC
- 37. Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. ArticlePubMedPDF
- 38. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623-50. ArticlePubMed
- 39. Chia CL, Mantoo SK, Tan KY. 'Start to finish trans-institutional transdisciplinary care': a novel approach improves colorectal surgical results in frail elderly patients. Colorectal Dis 2016;18:O43-50. ArticlePubMed
- 40. Karlsson E, Farahnak P, Franzen E, Nygren-Bonnier M, Dronkers J, van Meeteren N, et al. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery: a randomized controlled design. PLoS One 2019;14:e0219158.ArticlePubMedPMC
 , Jung Hoon Bae2
, Jung Hoon Bae2 , Jiseon Kim3
, Jiseon Kim3 , Eun Joo Yang4,*
, Eun Joo Yang4,* , Sang-Jae Park5
, Sang-Jae Park5 , In Kyu Lee2
, In Kyu Lee2 , on behalf of the Task Force Team for Development and Trial Application of Pre/Rehabilitation Protocol in GI Cancer Surgery
, on behalf of the Task Force Team for Development and Trial Application of Pre/Rehabilitation Protocol in GI Cancer Surgery 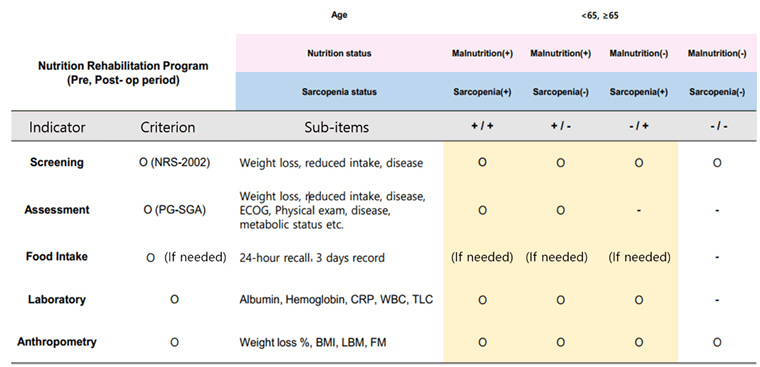


 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN

 ePub Link
ePub Link Cite
Cite

