Abstract
-
Purpose
Feeding catheter jejunostomy is a useful access route for early enteral nutrition during esophageal cancer surgery. However, it may lead to postoperative bowel obstruction associated with feeding jejunostomy (BOFJ). To prevent BOFJ, we introduced feeding catheter duodenostomy via the round ligament in 2018. This study aimed to compare the incidence of BOFJ and postoperative body weight changes between feeding catheter jejunostomy and duodenostomy.
-
Methods
A total of 109 patients who underwent thoracoscopic esophagectomy and gastric tube reconstruction for esophageal cancer at Kochi Medical School Hospital between March 2013 and November 2020 were included. Preoperative patient characteristics (age, sex, preoperative weight, body mass index, cancer stage, and preoperative treatment), surgical outcomes (operative time, blood loss, and postoperative complications [wound infection, pneumonia, anastomotic leakage, BOFJ]), and body weight changes at 1, 3, 6, and 12 months post-surgery were compared between the jejunostomy (J) and duodenostomy (D) groups.
-
Results
The D group consisted of 35 patients. No significant differences were observed between the groups regarding age, sex, weight, body mass index, cancer stage, operative time, postoperative complications, or duration of tube placement. However, the D group had a significantly lower rate of preoperative chemotherapy (45.7% vs. 78.4%, P=0.001) and lower operative blood loss (120 mL vs. 150 mL, P=0.046) than the J group. All 12 cases of BOFJ occurred in the J group. Furthermore, the D group experienced a significantly lower weight loss ratio at 1 month postoperatively (93.9% vs. 91.8%, P=0.039).
-
Conclusion
In thoracoscopic esophagectomy, feeding duodenostomy may prevent bowel obstruction and reduce early postoperative weight loss without increasing operative time compared with feeding catheter jejunostomy.
-
Keywords: Duodenostomy; Enteral nutrition; Esophageal cancer; Esophagectomy; Jejunostomy
Graphical abstract
Introduction
Background
Esophagectomy for esophageal cancer is a highly invasive procedure that involves lymph node dissection in the cervical, thoracic, and abdominal regions. Although minimally invasive thoracoscopic surgery has become more common in recent years [
1], the complication rate remains high [
2]. Notably, anastomotic leakage necessitates prolonged fasting and nutritional management. Even in the absence of anastomotic leakage, patients may require enteral nutrition at home because of nutritional deficiencies and weight loss resulting from decreased food intake [
3,
4]. Feeding jejunostomy is a valuable method for providing early enteral nutrition during the perioperative period for esophageal cancer [
5]. However, bowel obstruction associated with feeding jejunostomy (BOFJ), which results from bending, adhesion, or torsion of the intestinal tract around the catheter, is a common complication [
6]. To address this issue, we introduced feeding duodenostomy in 2018, in which a feeding tube is inserted through the duodenum via the round ligament.
We compared the effects of jejunostomy and duodenostomy on the incidence of BOFJ and postoperative weight changes in esophageal cancer surgery.
Methods
Ethics statement
This study was approved by the Institutional Review Board of Kochi Medical School Hospital (No. ERB-104180). The requirement for informed consent was waived.
Study design
This retrospective cohort study compared the effects of two interventions—feeding tube insertion into the jejunum versus the duodenum—on the incidence of BOFJ during the perioperative period of esophageal cancer.
Setting
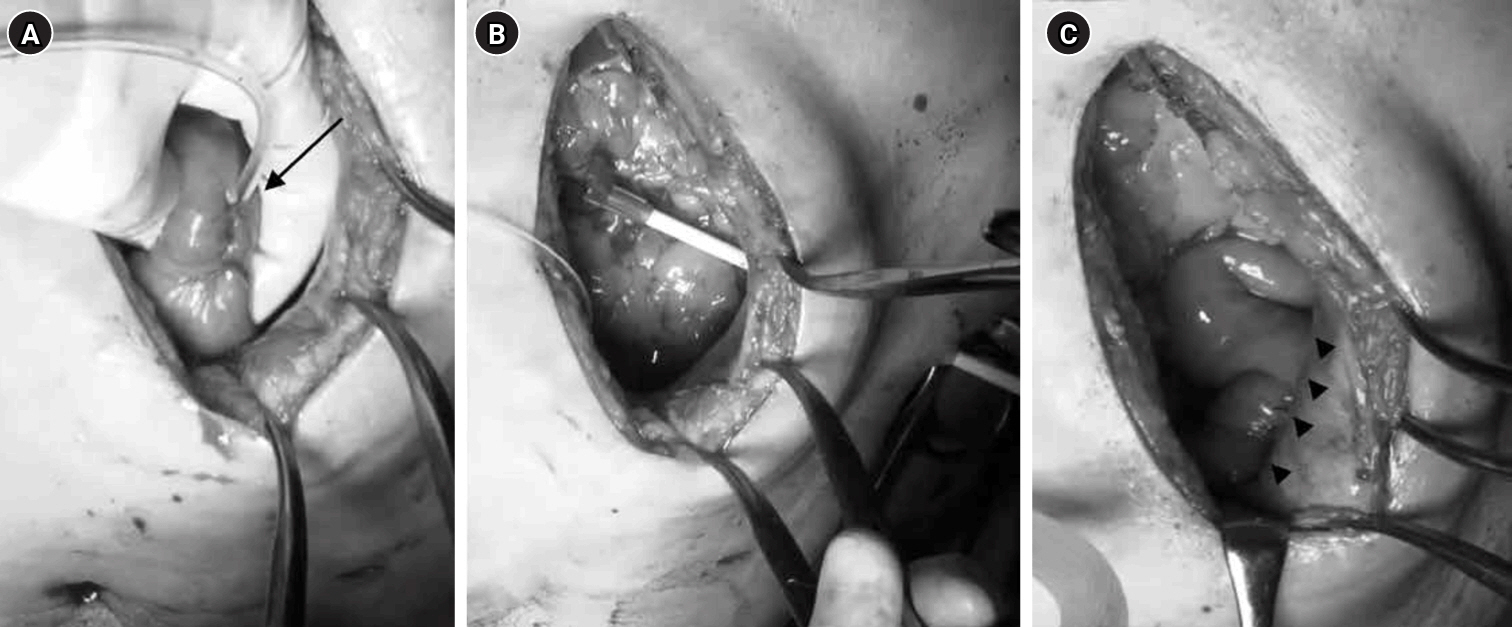
This study was conducted between March 2013 and November 2020. Tube became the standard method after August 2018. The feeding tube was placed under direct vision following gastric tube reconstruction. For jejunostomy, a 9-Fr tube was inserted 30 cm into the jejunum using a Seldinger kit approximately 25–30 cm from the Treitz ligament; the puncture site was secured with one purse-string suture and three Witzel stitches (
Fig. 1A). The tube was then guided out of the abdominal wall using a Seldinger kit (
Fig. 1B), and the abdominal wall along with the puncture site was fixed with four stitches. The jejunum on the anorectal side was sutured to the abdominal wall so that it formed a long axis of approximately 4 cm (
Fig. 1C).
For duodenostomy, following Kocher mobilization, a tube was inserted into the descending portion of the duodenum in a manner similar to the jejunostomy technique (
Fig. 2A), and the puncture site was buried. The round ligament of the liver was ligated and trimmed at the umbilicus, and the tube was guided out through the fatty tissue of the round ligament using a Seldinger kit (
Fig. 2B). Finally, the abdominal wall and the area around the duodenal puncture site were sutured and secured using the round ligament (
Fig. 2C). Continuous enteral nutrition was initiated at 20 mL/hr on the day following surgery, gradually increasing to 40–80 mL/hr until oral intake commenced. Once oral intake began, patients were instructed to self-administer 200 mL of nutritional supplements intermittently three times a day over 2 to 3 hours, continuing at home after discharge.
A total of 109 patients who underwent thoracoscopic esophagectomy and gastric tube reconstruction for esophageal cancer between March 2013 and November 2020 were included.
Variables
Preoperative baseline characteristics included age, sex, weight, body mass index, cancer stage, and preoperative treatment. Outcome variables comprised surgical outcomes (operative time and blood loss), postoperative complications (wound infection, pneumonia, anastomotic leakage, and BOFJ), and body weight at 1, 3, 6, and 12 months after surgery.
Data sources/measurement
Patients’ food intake was assessed during outpatient interviews 1 month or more after surgery. The feeding tube was removed once patients could consume sufficient food or nutritional supplements orally. Body weight was recorded at 1, 3, 6, and 12 months postoperatively, and these outcome variables were compared between the two groups.
Bias
As all eligible patients from a single institution during the study period were included, selection bias was not an issue.
Study size
No sample size estimation was performed because the study encompassed the entire target population.
Statistical methods
The chi-square test and Mann-Whitney test were employed for statistical analysis, with P<0.05 considered statistically significant.
Results
Participants
The median age was 68 years, with 74 patients in the jejunostomy (J) group and 35 patients in the duodenostomy (D) group (
Table 1). Among comorbidities, 17 patients (15.6%) had diabetes mellitus, 47 (43.1%) had hypertension, and 24 (22.0%) had cardiovascular disease. Posterior mediastinal route reconstruction was performed in 110 patients, and pneumonia and anastomotic leakage were observed in 15 patients (13.0%) each. Trends in nutritional doses up to 21 days postoperatively are presented in
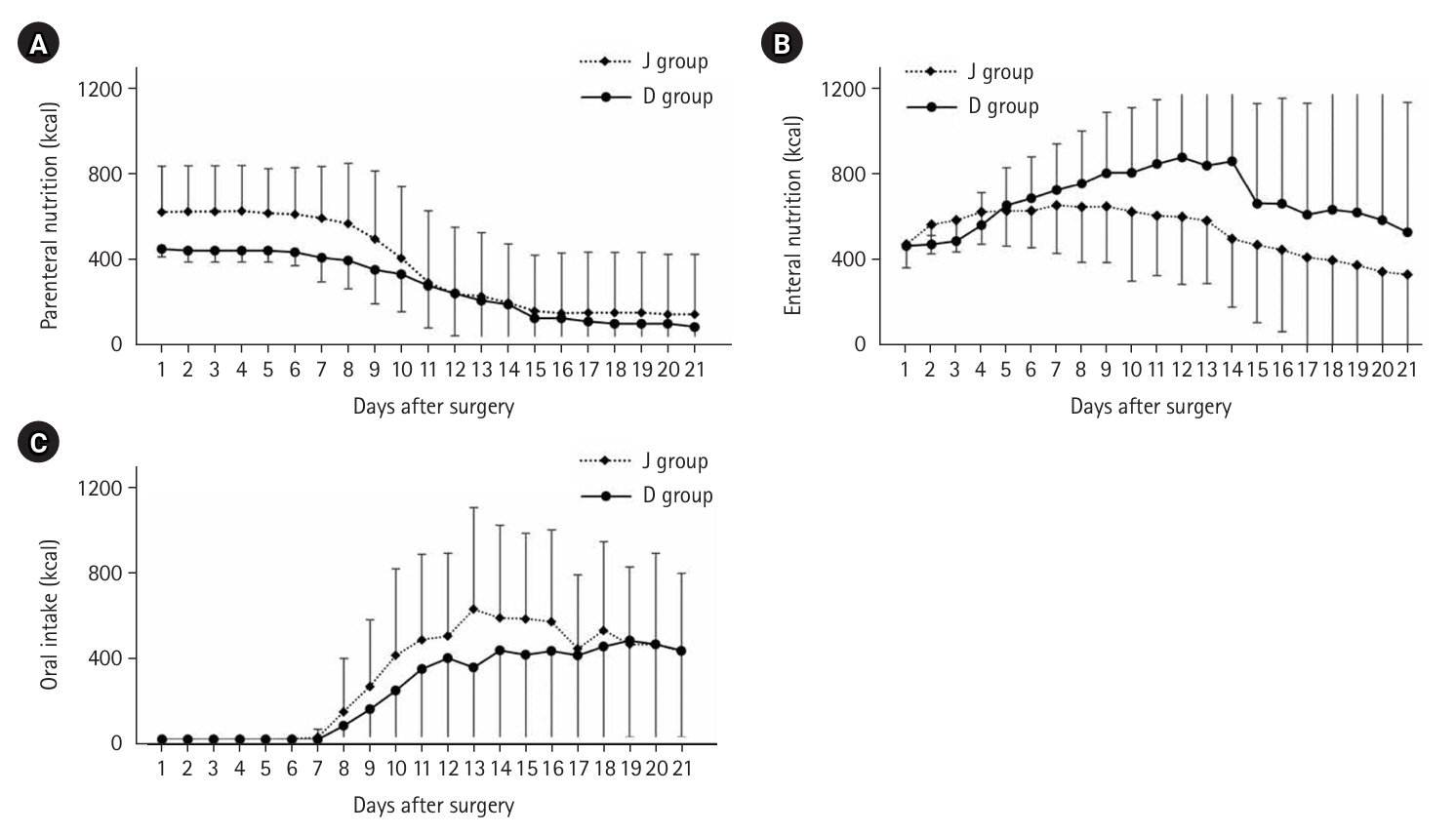
Fig. 3.
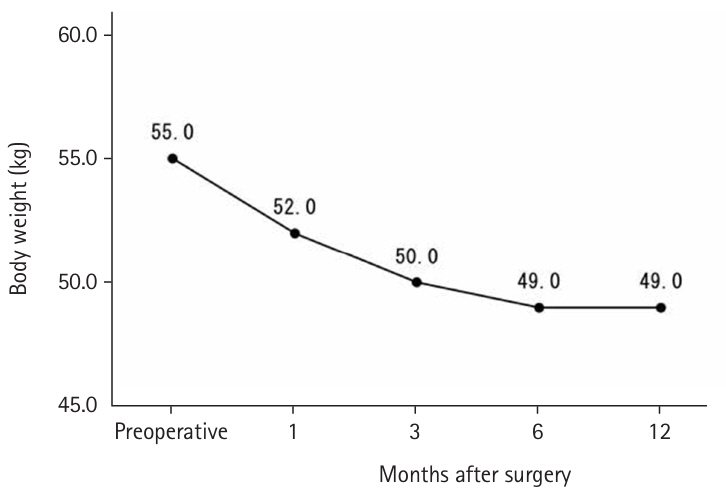
Until May 2018, high-calorie infusions were administered via a postoperative central venous catheter; as a result, the J group tended to receive more intravenous nutrition for up to 10 days postoperatively and less enteral nutrition after 8 days compared with the D group. The median preoperative weight was 55.0 kg, which decreased to 50.0 kg at 3 months postoperatively, with only slight further decreases at 6 and 12 months (
Fig. 4).
The median duration of feeding tube placement was 78 days. The shortest duration was 6 days postoperatively, observed in a patient who required emergency surgery for strangulated bowel obstruction. The longest duration was 376 days, as one patient requested extended use due to dysphagia and aspiration following head and neck cancer surgery. There were 12 cases (11.0%) of BOFJ, all occurring exclusively in the J group. The median time to BOFJ onset was 211 days post-surgery, with the longest interval being 1,450 days. Additionally, one patient in the D group developed a peri-pancreatic abscess with a pancreatic fistula, as indicated by a high amylase level in the drainage.
Comparisons between the groups revealed no significant differences in age, gender, weight, body mass index, cancer stage, operative time, postoperative complications, or duration of tube placement. However, the D group had significantly less preoperative chemotherapy (45.7% vs. 78.4%, P=0.001) and lower operative blood loss (120 mL vs. 150 mL, P=0.046) compared with the J group (
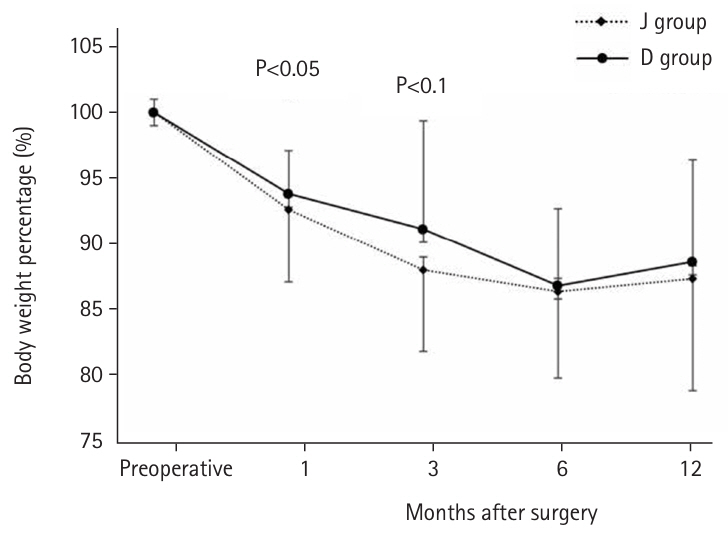
Table 2). Regarding the postoperative weight-to-preoperative weight ratio (set at 100%) (
Fig. 5), the D group exhibited a significantly lower weight loss rate at 1 month postoperatively (93.9% vs. 91.8%, P=0.039). Although the rate remained lower at 3 months (90.0% vs. 87.3%, P=0.077), no difference was observed after 6 months. When excluding patients with BOFJ, there was no significant difference in the weight loss rate at 1 month (93.9% vs. 93.1%, P=0.244). At 3 months, a trend toward a lower weight loss rate in the D group persisted (90.0% vs. 88.6%, P=0.056).
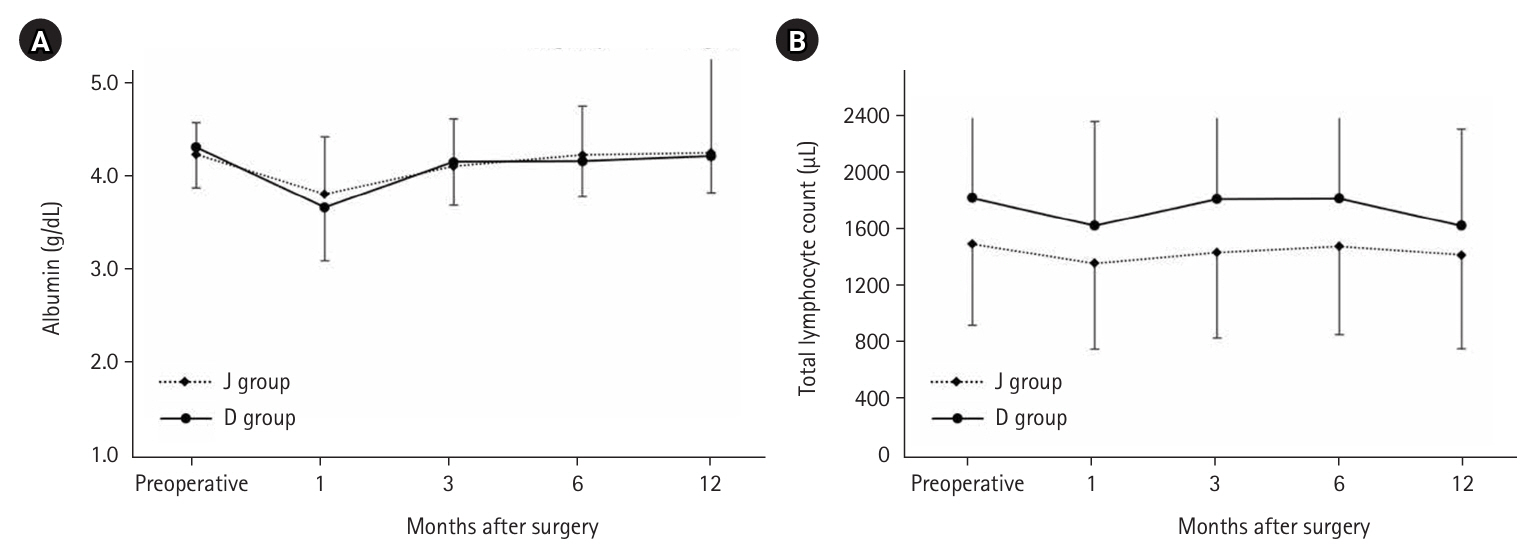
There was no significant difference in serum albumin levels between the two groups; however, the total lymphocyte count was higher in the D group (
Fig. 6). Additionally, the incidence of BOFJ did not differ significantly between patients who received preoperative chemotherapy and those who did not (9.5% vs. 14.3%, P=0.517).
Discussion
Key results
The present study suggests that tube duodenostomy can prevent BOFJ and weight loss in the early postoperative period, without prolonging the operative time compared to jejunostomy.
Interpretation/comparison with previous studies
Early postoperative rehabilitation and enteral nutrition, as advocated by the Enhanced Recovery After Surgery protocol, are recommended during the perioperative period for esophageal cancer [
7]. However, clear guidelines regarding the optimal access route for enteral nutrition remain lacking. With jejunostomy, it is recommended that the catheter be fixed to the abdominal wall to prevent bending of the intestinal tract around the catheter—a factor that may lead to obstruction due to bending, adhesion, or torsion. In some cases, the intestinal tract on the anorectal side may fall into the space above the abdominal wall fixation site, further predisposing it to torsion [
8].
We also secured the jejunum along its long axis on the anorectal side of the tube. Despite this, we encountered cases where the fixation thread dislodged when using absorbable sutures, as well as instances of torsion even with non-absorbable threads. In addition, inadequate abdominal wall fixation on the oral side of the tube insertion site may have contributed to tube bending. A previous study reported that a tube entry site located near the midline of the abdominal wall was correlated with BOFJ [
6]. This association may be due to the laparoscopic abdominal lymph node dissection followed by gastric tube creation and jejunostomy through a small incision, which positions the tube entry site near the midline and creates a non-adherent space on the left side into which the jejunum on the anal side can herniate, mimicking an internal hernia.
Kamada et al. [
9] performed a button-type jejunostomy in the jejunum 20 cm distal to the Treitz ligament and measured the distance from the button to the umbilicus via computed tomography. They found that a longer vertical distance was associated with intestinal obstruction compared with cases without obstruction.
Their procedure, which involved hand-assisted laparoscopic surgery with a small incision just below the xiphoid process, suggested that placing the jejunostomy in the upper abdomen increases the angle from the Treitz ligament to the jejunostomy, leading to flexion. Based on these findings, the optimal jejunostomy site should be as far left lateral as possible at the level of the umbilicus and secured to the abdominal wall with non-absorbable sutures along the long axis of the anal jejunum to prevent internal hernia. Employing a laparoscopic approach to position the jejunostomy on the left lateral side may be more suitable than direct visualization through a small laparotomy—a topic warranting further investigation.
Duodenostomy, which involves inserting a tube through the antrum of the stomach via the round ligament of the liver, is less likely to result in internal hernia or torsion than jejunostomy. This is because the space above the abdominal wall is shielded by the liver, and the horizontal portion of the duodenum is fixed to the retroperitoneum, thereby preventing torsion or internal herniation of the distal intestine [
10-
12]. In our study, no cases of BOFJ were observed in the D group, and the rate of weight loss at 1 to 3 months postoperatively was lower compared to the J group. These findings suggest that duodenostomy may reduce flexion and torsion, facilitating smoother food passage.
Oya et al. [
10] inserted a tube through the duodenum just below the pyloric ring, whereas Kawai et al. [
11] and Huang et al. [
12] placed the tube through the antrum of the reconstructed gastric tube. In our practice, we generally employed the posterior mediastinal route; however, because the antrum of the elevated gastric tube was located near the esophageal hiatus, it was challenging to insert the tube and secure the round ligament. Consequently, we performed Kocher's mobilization of the duodenum and inserted the tube through its descending portion. In this configuration, the distance between the abdominal wall and the duodenum is longer than that in posterior sternal route reconstruction, which may predispose patients with minimal fat tissue around the round ligament to leakage of intestinal fluid. Additionally, if edema develops in the descending portion of the duodenum due to Kocher's mobilization, duodenal puncture, or suturing, it may lead to inflammation at the puncture site or edema of the Vater's papilla. Therefore, inserting the tube through the antrum of the gastric tube via the posterior sternal route might be a better option.
There is ongoing debate regarding the necessity of a feeding tube for all patients. Akiyama et al. [
13] found no significant differences in infectious complications or hospital stay duration between patients receiving parenteral nutrition combined with jejunostomy and those receiving PPN alone. Similarly, Koterazawa et al. [
14] reported no difference in weight loss at 3 months post-surgery based on the presence or absence of jejunostomy; however, 11% of patients in the J group experienced intestinal obstruction. Their multivariate analysis identified age 75 years or older, preoperative treatment, anastomotic leakage, and pneumonia as factors associated with the need for long-term jejunostomy. In our study, 48 patients (41.7%) were aged 70 years or older, and many were on antihypertensive or antithrombotic medications, suggesting that early postoperative administration of these drugs via jejunostomy may offer an advantage.
This retrospective study involved a small number of cases, and the perioperative nutritional doses varied among patients. Furthermore, the exact amount and duration of enteral nutrition were not strictly defined, making it difficult to quantify the nutritional benefits. Future prospective studies with standardized nutritional dosing and duration are needed to more accurately assess the benefits of duodenostomy versus jejunostomy.
Conclusion
Patients who underwent duodenostomy experienced no bowel obstruction and demonstrated reduced early postoperative weight loss without an increase in operative time. These results suggest that feeding duodenostomy is a safer option for enteral nutrition in patients undergoing esophagectomy. Despite the limitations of a retrospective design and variability in nutritional dosing, our findings support further prospective investigations to validate these results and refine feeding strategies for improved outcomes in esophageal cancer surgery.
Authors’ contribution
Conceptualization: KH. Data curation: HK. Methodology/formal analysis/validation: HK, KY, TN, KH. Project administration: KH. Funding acquisition: Not applicable. Writing–original draft: HK. Writing–review & editing: HK, KY, TN, KH. All authors read and approved the final manuscript.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
The authors would like to thank Professor Satoru Seo for his invaluable guidance and final revision of the manuscript.
Supplementary materials
None.
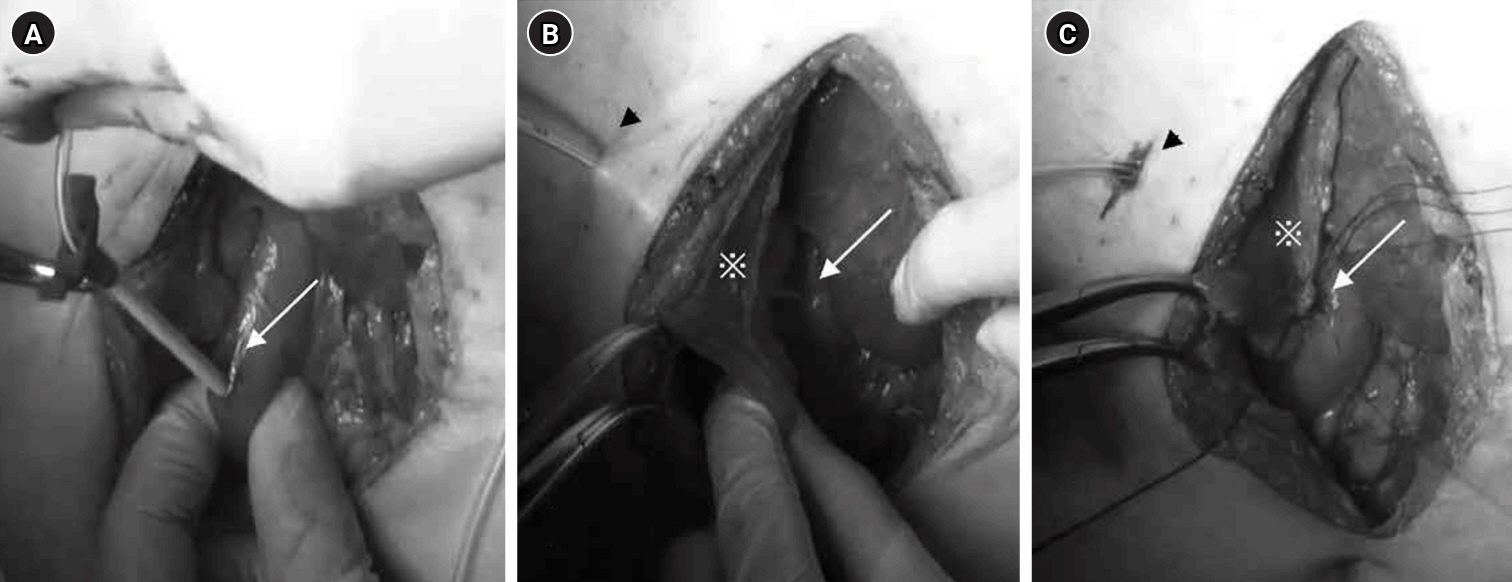
Fig. 1.Surgical technique for jejunostomy. (A) The jejunal puncture site for the 9-Fr tube (arrow) was secured with one drawstring suture and three Witzel sutures. (B) The tube was guided out of the abdominal wall. (C) The anorectal portion of the jejunum was fixed to the abdominal wall over a length of approximately 4 cm (arrowheads).
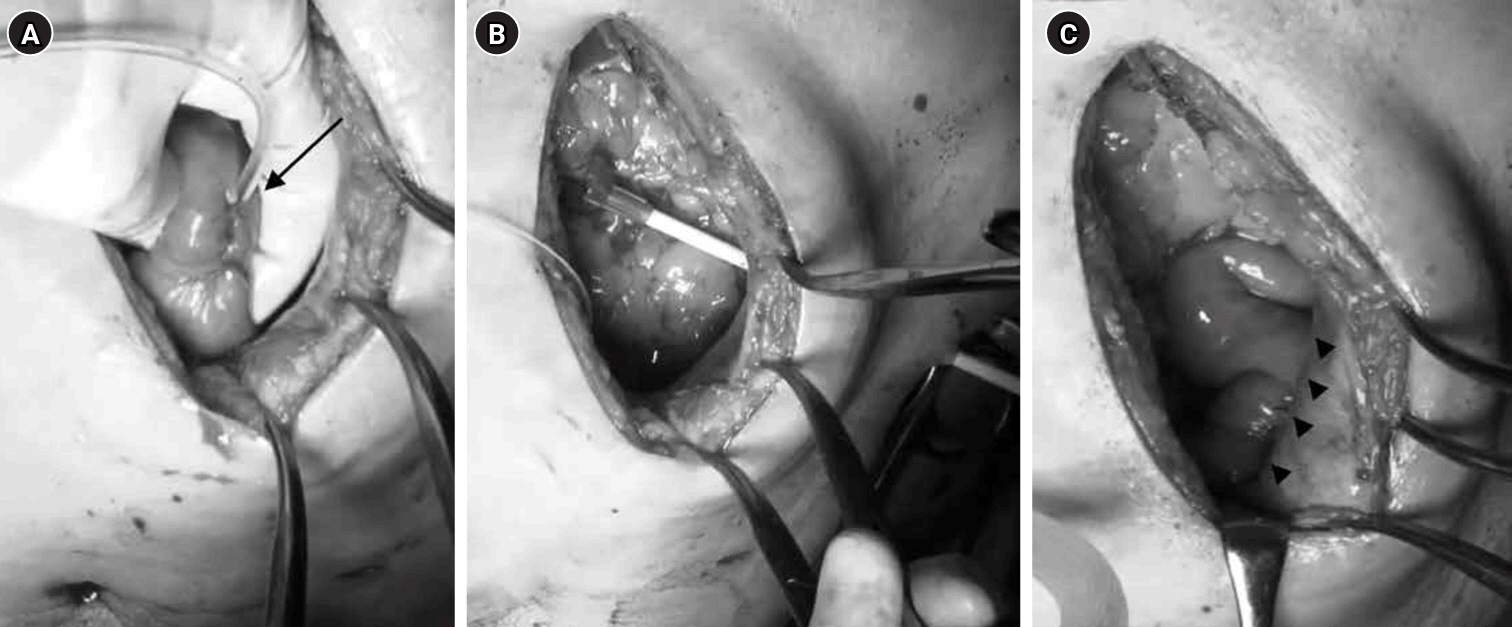
Fig. 2.Surgical technique for tube duodenostomy. (A) The tube was inserted into the descending duodenal leg (arrow) and implanted. (B) After guiding the tube out of the abdominal wall (arrowhead), the hepatic round ligament (*) was trimmed to reach the duodenal entry point of the tube (arrow). (C) The hepatic round ligament (*), the abdominal wall (arrowhead), and the area around the duodenal entry site (arrow) were sutured and fixed.
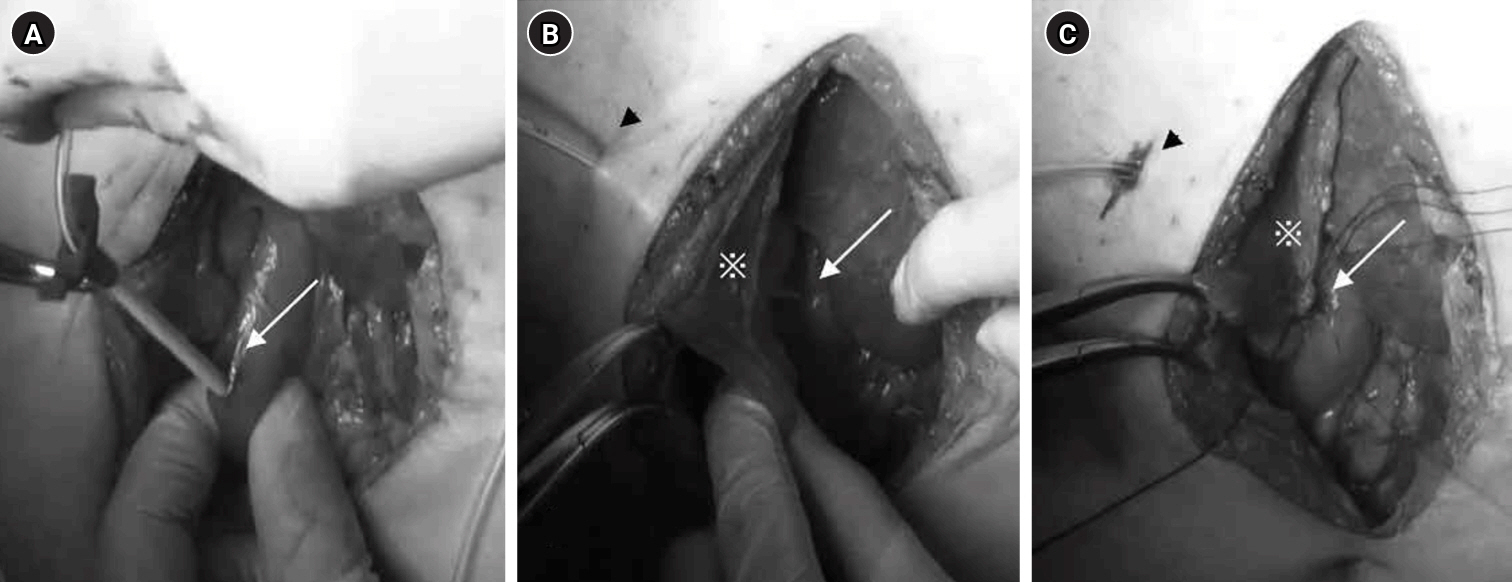
Fig. 3.Changes in the amount of energy administered for postoperative nutrition. (A) Intravenous nutrition, (B) enteral nutrition, and (C) oral intake. J group, jejunostomy group; D group, duodenostomy group.
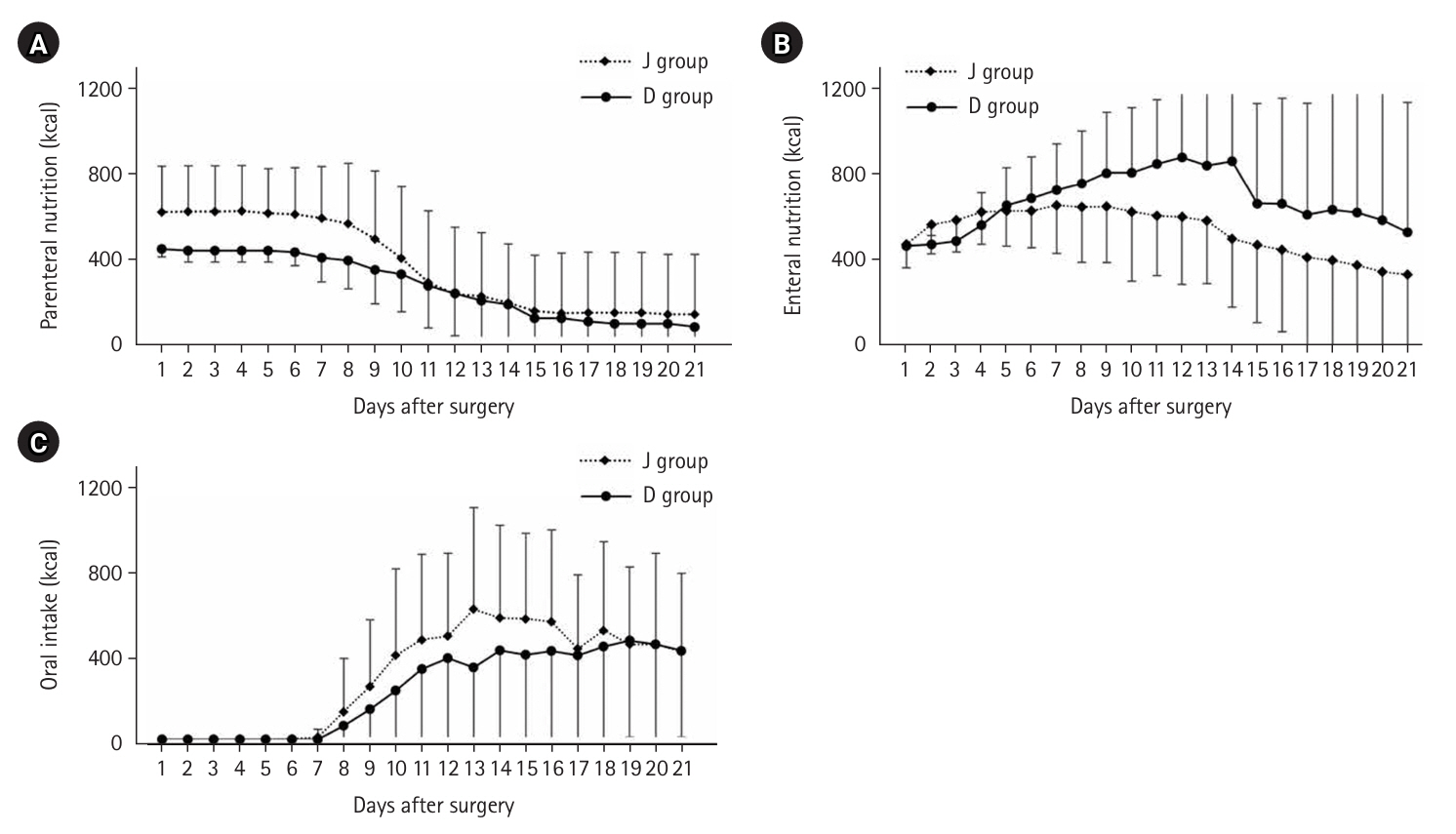
Fig. 4.Preoperative and postoperative weight change (kg).
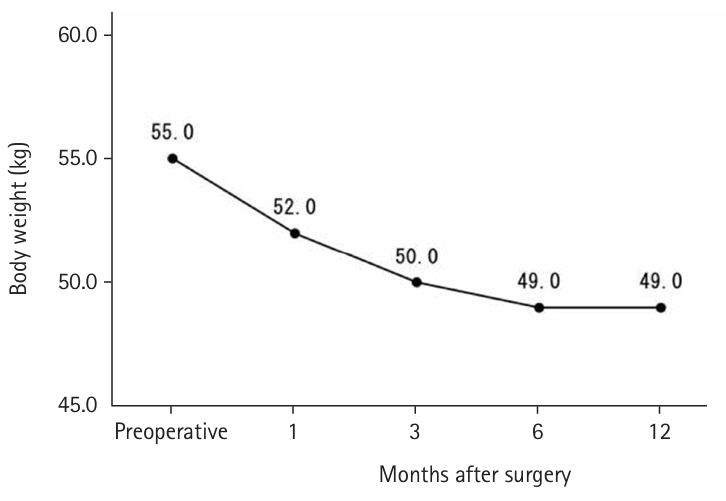
Fig. 5.Preoperative and postoperative body weight ratio (%). J group, jejunostomy group; D group, duodenostomy group.
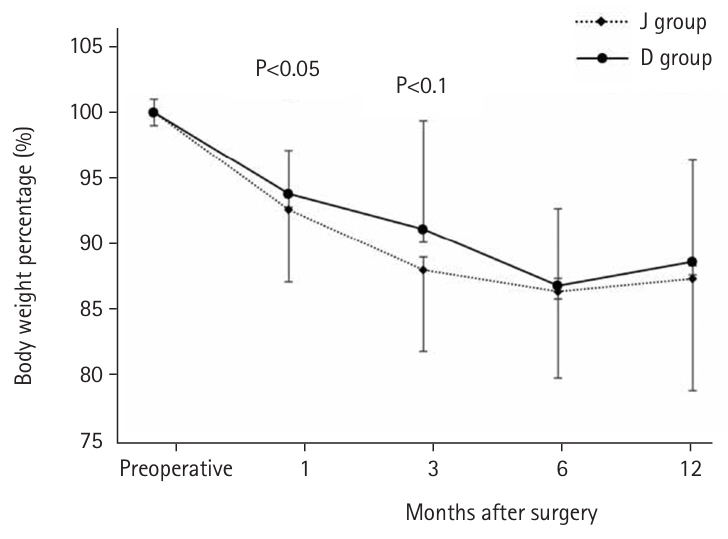
Fig. 6.Serum albumin and total lymphocyte count. J group, jejunostomy group; D group, duodenostomy group.
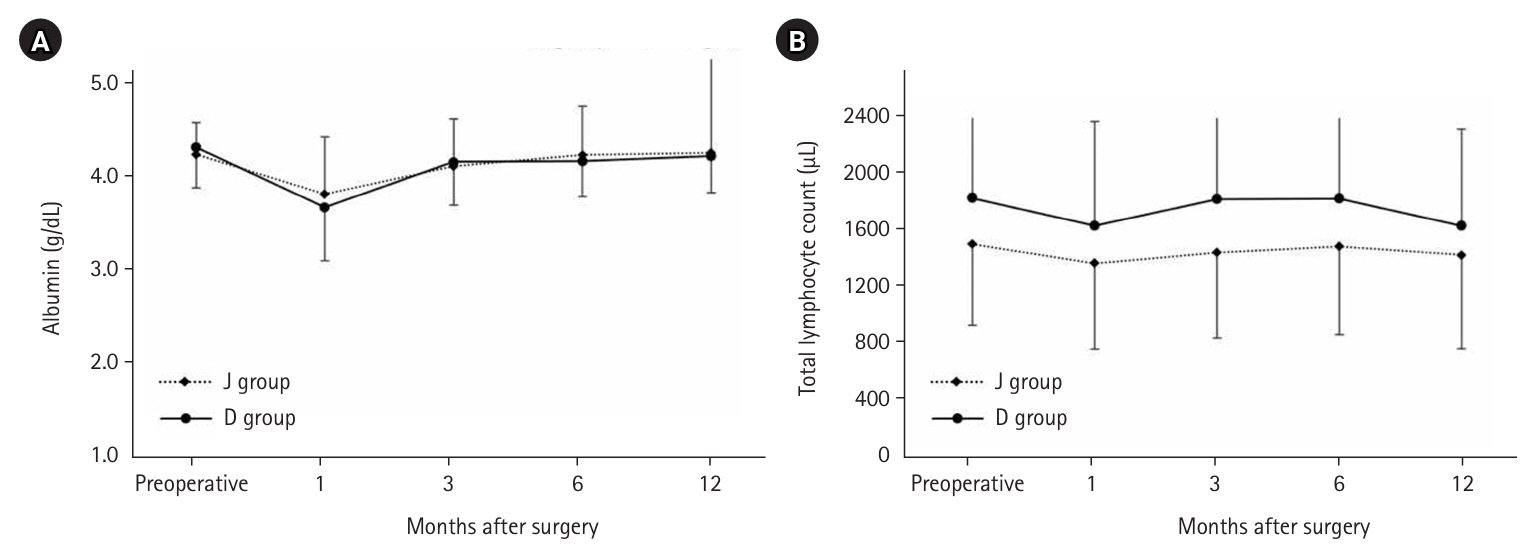
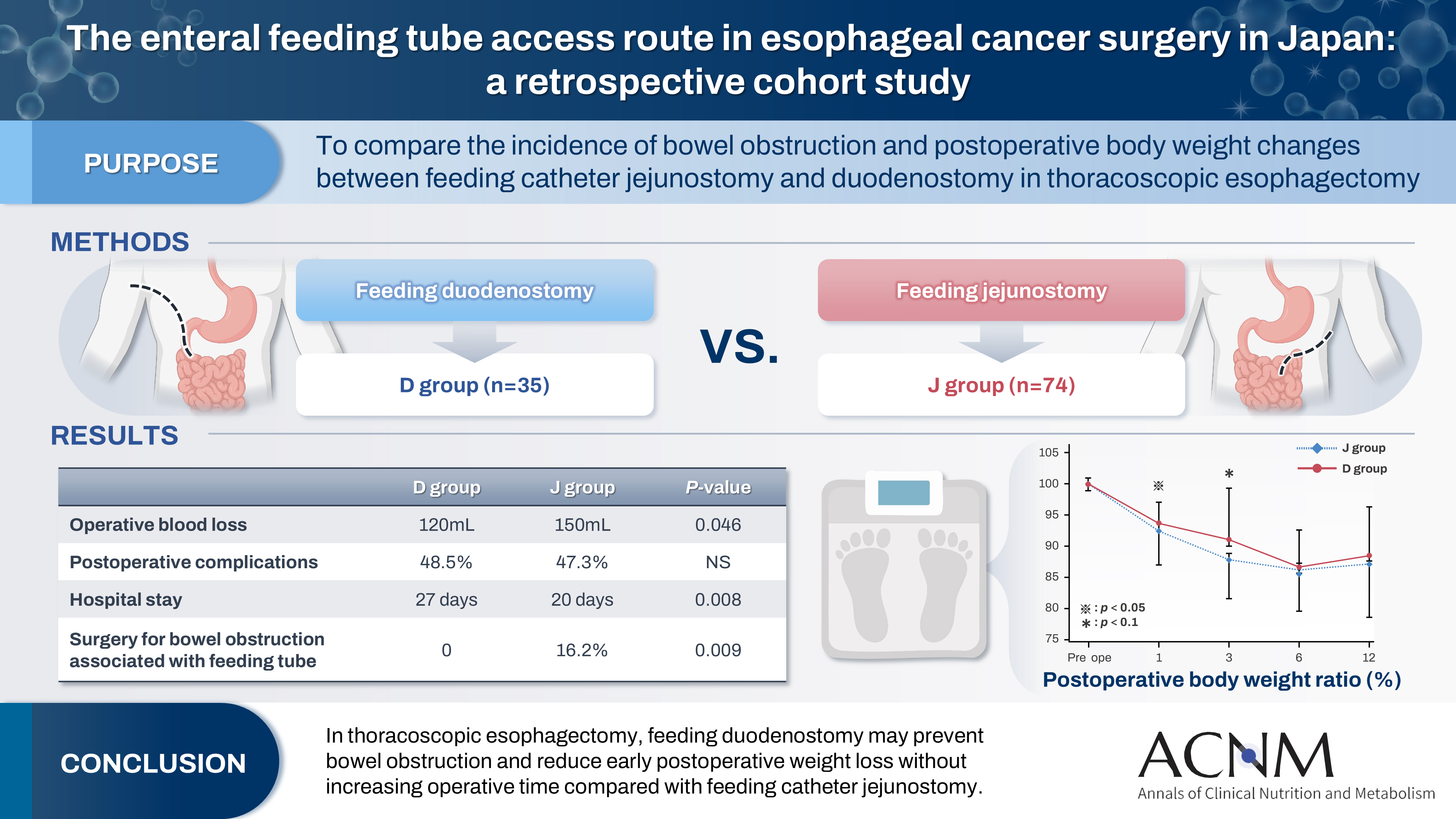
Table 1.Characteristics of the 109 patients who underwent thoracoscopic esophagectomy for esophageal cancer
|
Characteristic |
Number (%) |
|
Male sex |
84 (77.1) |
|
Age (yr), median (range) |
68 (43–91) |
|
cT 1/2/3/4 |
41/12/49/7 |
|
cN 0/1/2/3 |
40/43/15/11 |
|
cM 0/1 |
94/15 |
|
cStage I/II/III/IV |
39/18/29/23 |
|
Diabetes mellites |
17 (15.6) |
|
Hypertension |
47 (43.1) |
|
Cardiovascular disease |
24 (22.0) |
|
Preoperative body weight (kg), median (range) |
55.0 (30.7–78.0) |
|
Preoperative body mass index (kg/m2), median (range) |
21.3 (14.0–30.0) |
|
Neoadjuvant chemotherapy |
74 (67.9) |
|
History of radiation therapy |
12 (11.0) |
|
Operative time (min), median (range) |
607 (379–859) |
|
Blood loss (mL), median (range) |
150 (10–1,600) |
|
Postoperative complications |
|
|
Pneumonia |
14 (12.8) |
|
Anastomotic leakage |
15 (13.8) |
|
Wound infection |
23 (21.1) |
|
Hospital stay (day), median (range) |
21 (10–201) |
|
Type of feeding tube, duodenostomy/jejunostomy |
35/74 |
|
Duration until feeding catheter removal (day), median (range) |
78 (6–376) |
|
Surgery for bowel obstruction associated with feeding tube |
12 (11.0) |
Table 2.Comparison of the outcomes between the two groups
|
Variable |
Duodenostomy (n=35) |
Jejunostomy (n=74) |
P-value |
|
Male sex |
25 (71.4) |
59 (79.7) |
0.336 |
|
Age (yr), median (range) |
70 (49–91) |
67 (43–82) |
0.250 |
|
cT 1/2/3/4 |
16/4/11/4 |
25/8/38/3 |
0.117 |
|
cN 0/1/2/3 |
20/7/6/2 |
20/36/9/9 |
0.007 |
|
cM 0/1 |
29/6 |
65/9 |
0.481 |
|
Stage I/II/III/IV |
16/5/7/7 |
23/13/22/16 |
0.462 |
|
Diabetes mellites |
4 (11.4) |
13 (17.6) |
0.574 |
|
Hypertensions |
18 (51.4) |
29 (39.2) |
0.228 |
|
Cardiovascular disease |
9 (25.7) |
15 (20.3) |
0.522 |
|
Preoperative body weight (kg), median (range) |
52.9 (30.7–77.4) |
55.3 (39.9–78.0) |
0.525 |
|
Preoperative body mass index (kg/m2), median (range) |
21.9 (14.0–27.7) |
21.2 (14.1–30.0) |
0.987 |
|
Neoadjuvant chemotherapy |
16 (45.7) |
58 (78.4) |
0.001 |
|
History of radiation therapy |
5 (14.3) |
7 (9.5) |
0.517 |
|
Operative time (min), median (range) |
593 (379–694) |
611 (456–859) |
0.115 |
|
Blood loss (mL), median (range) |
120 (30–950) |
150 (10–1,600) |
0.046 |
|
Postoperative complications |
|
|
|
|
Pneumonia |
4 (11.4) |
10 (13.5) |
1.000 |
|
Anastomotic leakage |
6 (17.1) |
9 (12.2) |
0.555 |
|
Wound infection |
7 (20.0) |
16 (21.6) |
0.846 |
|
Hospital stay (day), median (range) |
27 (13–144) |
20 (10–201) |
0.008 |
|
Duration until feeding catheter removal (day), median (range) |
67 (46–376) |
78 (6–316) |
0.379 |
|
Surgery for bowel obstruction associated with feeding tube |
0 |
12 (16.2) |
0.009 |
References
- 1. Osugi H, Takemura M, Higashino M, Takada N, Lee S, Kinoshita H. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. ArticlePubMedPDF
- 2. Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. ArticlePubMed
- 3. Ouattara M, D'Journo XB, Loundou A, Trousse D, Dahan L, Doddoli C, et al. Body mass index kinetics and risk factors of malnutrition one year after radical oesophagectomy for cancer. Eur J Cardiothorac Surg 2012;41:1088-93. ArticlePubMed
- 4. Donohoe CL, Healy LA, Fanning M, Doyle SL, Hugh AM, Moore J, et al. Impact of supplemental home enteral feeding postesophagectomy on nutrition, body composition, quality of life, and patient satisfaction. Dis Esophagus 2017;30:1-9. Article
- 5. Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, Ruurda JP, van Hillegersberg R, Soeters PB, et al. Routes for early enteral nutrition after esophagectomy: a systematic review. Clin Nutr 2015;34:1-6. ArticlePubMed
- 6. Kitagawa H, Namikawa T, Munekage M, Fujisawa K, Munekage E, Kawanishi Y, et al. Analysis of factors associated with weight loss after esophagectomy for esophageal cancer. Anticancer Res 2016;36:5409-412. ArticlePubMed
- 7. Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. ArticlePubMed
- 8. Shiraishi O, Kato H, Iwama M, Hiraki Y, Yasuda A, Peng YF, et al. A simple, novel laparoscopic feeding jejunostomy technique to prevent bowel obstruction after esophagectomy: the "curtain method". Surg Endosc 2020;34:4967-74. ArticlePubMedPDF
- 9. Kamada T, Ohdaira H, Takeuchi H, Takahashi J, Marukuchi R, Ito E, et al. Vertical distance from navel as a risk factor for bowel obstruction associated with feeding jejunostomy after esophagectomy: a retrospective cohort study. BMC Gastroenterol 2020;20:354.ArticlePubMedPMCPDF
- 10. Oya H, Koike M, Iwata N, Kobayashi D, Torii K, Niwa Y, et al. Feeding duodenostomy decreases the incidence of mechanical obstruction after radical esophageal cancer surgery. World J Surg 2015;39:1105-10. ArticlePubMedPDF
- 11. Kawai R, Abe T, Uemura N, Fukaya M, Saito T, Komori K, et al. Feeding catheter gastrostomy with the round ligament of the liver prevents mechanical bowel obstruction after esophagectomy. Dis Esophagus 2017;30:1-8. Article
- 12. Huang K, Wu B, Ding X, Xu Z, Tang H. Post-esophagectomy tube feeding: a retrospective comparison of jejunostomy and a novel gastrostomy feeding approach. PLoS One 2014;9:e89190.ArticlePubMedPMC
- 13. Akiyama Y, Iwaya T, Endo F, Nikai H, Sato K, Baba S, et al. Evaluation of the need for routine feeding jejunostomy for enteral nutrition after esophagectomy. J Thorac Dis 2018;10:6854-62. ArticlePubMedPMC
- 14. Koterazawa Y, Oshikiri T, Hasegawa H, Yamamoto M, Kanaji S, Yamashita K, et al. Routine placement of feeding jejunostomy tube during esophagectomy increases postoperative complications and does not improve postoperative malnutrition. Dis Esophagus 2020;33:doz021.ArticlePubMedPDF
 , Keiichiro Yokota
, Keiichiro Yokota , Tsutomu Namikawa
, Tsutomu Namikawa , Kazuhiro Hanazaki
, Kazuhiro Hanazaki







 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN


 ePub Link
ePub Link Cite
Cite

