Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > J Clin Nutr > Volume 9(2); 2017 > Article
- Original Article Determination of the Stress Factor Calculated from the Changes in the Measured Resting Energy Expenditure with Indirect Calorimetry in Patients Undergoing Pancreaticoduodenectomy
- Seon Hyeong Kim1,2, Baik Hwan Cho1,3, Sook Bae Kim2, Mi Jin Jeong1, Hee Chul Yu1,3
- 췌십이지장절제술 환자의 간접열량측정기로 측정한 휴식대사량 변화를 이용한 스트레스 계수의 산정
- 김선형1,2, 조백환1,3, 김숙배2, 정미진1, 유희철1,3
-
Journal of the Korean Society for Parenteral and Enteral Nutrition 2017;9(2):62-67.
DOI: https://doi.org/10.15747/jcn.2017.9.2.62
Published online: December 31, 2017
Nutrition Support Team, Chonbuk National University Hospital, Jeonju, Korea
Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju, Korea
Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea
- Correspondence to Hee Chul Yu Department of Surgery, Chonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea Tel: +82-63-250-1604, Fax: +82-63-250-1604, E-mail: hcyu@jbnu.ac.kr
Copyright: © Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 6,579 Views
- 72 Download
Abstract
-
Purpose To predict the energy expenditure using the stress factor representing the ratio of the metabolic variation between pre-operation and post-operation in a pancreaticoduodenectomy (PD).
-
Methods This was a prospective study conducted on 17 patients (11 males and 6 females) who underwent PD at Chonbuk National University Hospital between March 2010 and October 2011. The rest energy expenditure was measured by indirect calorimetry 1 day before and 3 days after surgery. The height, weight, and fat free mass were also measured 1 day before surgery.
-
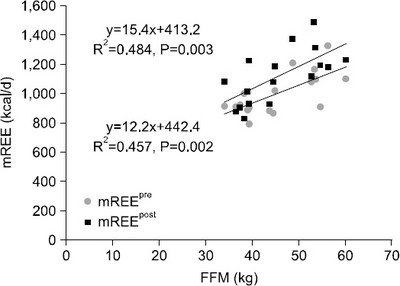
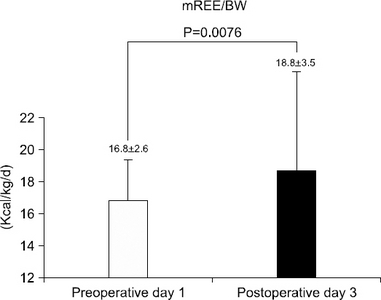
Results The mean measured rest energy expenditure 1 day before PD (mREEpre) and 3 days after PD (mREEpost) were significantly different (16.8±2.6 vs. 18.8±3.5 kcal/kg/d, P=0.0076). The stress factor, representing the ratio of the metabolic changes between pre- and post-PD, was 1.12±0.17. The recommended energy requirement for PD patients is estimated to be 23∼24 kcal/ideal body weight/d [determined from the measured preoperative rest energy expenditure (16.8±2.6 kcal/kg/d)×activity factor (1.2∼1.3)×stress factor (1.12)].
-
Conclusion PD patients maintained a hypermetabolic status and the applicable stress factor was 1.12.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
| Measurements | Data |
|---|---|
| Sex (male/female) | 11/6 |
| Age (y) | 62.2±12.8 |
| Weight (kg) | 60.1±8.7 |
| Height (cm) | 160.5±7.5 |
| Ideal body weight (kg)a | 56.5±5.6 |
| Fat free mass (kg) | 44.2±8.7 |
| BMI (kg/m2) | 23.2±2.5 |
| Underweightb | 1 (5.9) |
| Adequate | 5 (29.4) |
| Overweight | 6 (35.3) |
| Obese | 5 (29.4) |
| Diagnosis | |
| Pancreatic head carcinoma | 5 (29.4) |
| Ampulla of Vater carcinoma | 5 (29.4) |
| Distal common bile duct carcinoma | 7 (41.2) |
| Operation procedure | |
| Pylorus preserving pancreaticoduodenectomy | 11 (64.7) |
| Whipple’s procedure | 6 (35.3) |
| Nutritional status | |
| Well-nourished | 14 (82.4) |
| Moderate malnutrition | 3 (17.6) |
| Severe malnutrition | 0 (0) |
Values are presented as number only, mean±standard deviation, or number (%).
aIdeal body weight was calculated by squared meter height multiply with 22 in male (22×m2), 21 in female (21×m2).
bClassified according to the criteria of the World Health Organization Asia-Pacific, body mass index (BMI)<18.5 kg/m2 is underweight, BMI 18.5∼22.9 kg/m2 is adequate, BMI 23.0∼24.9 kg/m2 is overweight, BMI>25.0 kg/m2 is obese.
| Nutrition route | Preoperative day 1 | Postoperative day 3 |
|---|---|---|
| Only parenteral | 12 | 0 |
| Parenteral+oral | 0 | 14 |
| NPO | 5 | 3 |
| Energy intake (kcal/kg/d)a | 14.5±4.7 | 17.4±5.3 |
DISCUSSION
CONCLUSION
- 1. Kemper M, Weissman C, Hyman AI. Caloric requirements and supply in critically ill surgical patients. Crit Care Med 1992;20(3):344-8. ArticlePubMed
- 2. Auxiliadora Martins M, Menegueti MG, Nicolini EA, Picolo MF, Lago AF, Martins Filho OA, et al. Energy expenditure in critically ill surgical patients. Comparative analysis of predictive equation and indirect calorimetry. Acta Cir Bras 2011;26(Suppl2):51-6. Article
- 3. Pappas S, Krzywda E, McDowell N. Nutrition and pancreaticoduodenectomy. Nutr Clin Pract 2010;25(3):234-43. ArticlePubMedPDF
- 4. Lassen K, Kjaeve J, Fetveit T, Tranø G, Sigurdsson HK, Horn A, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247(5):721-9. PubMed
- 5. Wooley JA. Indirect calorimetry: applications in practice. Respir Care Clin N Am 2006;12(4):619-33. PubMed
- 6. Reilly JJ Jr, Gerhardt AL. Modern surgical nutrition. Curr Probl Surg 1985;22(10):1-81. Article
- 7. Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract 2007;22(4):377-88. ArticlePubMedPDF
- 8. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109(1-2):1-9. ArticlePubMedPMC
- 9. Schiesser M, Müller S, Kirchhoff P, Breitenstein S, Schäfer M, Clavien PA. Assessment of a novel screening score for nutritional risk in predicting complications in gastro-intestinal surgery. Clin Nutr 2008;27(4):565-70. ArticlePubMed
- 10. Cerantola Y, Grass F, Cristaudi A, Demartines N, Schäfer M, Hübner M. Perioperative nutrition in abdominal surgery: recommendations and reality. Gastroenterol Res Pract 2011;doi:10.1155/2011/739347. ArticlePDF
- 11. Prieto Reyes MA, Márquez Báez MA, Vázquez Márquez L, Redel del Pueyo J, Gordón del Río A, Arévalo Jiménez E. Nutritional status of patients undergoing digestive surgery. Nutr Hosp 1993;8(2):94-6. PubMed
- 12. Hill AG, Hill GL. Metabolic response to severe injury. Br J Surg 1998;85(7):884-90. ArticlePubMedPDF
- 13. Jones MO, Pierro A, Hammond P, Lloyd DA. The metabolic response to operative stress in infants. J Pediatr Surg 1993;28(10):1258-62. ArticlePubMed
- 14. Reid CL. Nutritional requirements of surgical and critically-ill patients: do we really know what they need? Proc Nutr Soc 2004;63(3):467-72. ArticlePubMed
- 15. Sax HC, Souba WW. Nutritional goals and macronutrient requirement. The A.S.P.E.N nutrition support practice manual. 2nd ed. Silver Spring, MD: ASPEN; 1998. p. 2-3.
- 16. Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr 1979;3(6):452-6. ArticlePubMed
- 17. Rutten P, Blackburn GL, Flatt JP, Hallowell E, Cochran D. Determination of optimal hyperalimentation infusion rate. J Surg Res 1975;18(5):477-83. ArticlePubMed
- 18. Farhi LE, Rahn H. Gas stores of the body and the unsteady state. J Appl Physiol 1955;7(5):472-84. ArticlePubMed
- 19. Sasaki M, Okamoto H, Johtatsu T, Kurihara M, Iwakawa H, Tanaka T, et al. Resting energy expenditure in patients undergoing pylorus preserving pancreatoduodenectomies for bile duct cancer or pancreatic tumors. J Clin Biochem Nutr 2011;48(3):183-6. ArticlePubMedPMC
- 20. Frankentfield D. Energy dynamics. In: Matarese LE, Gottschlich MM, editors. Contemporary nutrition support practice: a clinical guide. Philadelphia: WB Saunders Co; 1998. p. 88-9.
- 21. Merritt R, DeLegge MH, Holcombe B, Mueller C, Ochoa J, Smith KR, et al. Fundamentals of nutrition support practice and management. The A.S.P.E.N nutrition support practice manual 2nd ed. Silver Spring, MD: ASPEN; 1998;45.
- 22. Sánchez Álvarez C, Zabarte Martínez de Aguirre M, BordejéLaguna L; Metabolism and Nutrition Working Group of the Spanish Society of Intensive Care Medicine and Coronary units. Guidelines for specialized nutritional and metabolic support in the critically-ill patient: update. Consensus SEMICYUC-SENPE: gastrointestinal surgery. Nutr Hosp 2011;26(Suppl2):41-5. PubMed
- 23. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr 2009;28(4):378-86. PubMed
- 24. Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care 2009;54(4):509-21. PubMed
- 25. Ishikawa M, Nishioka M, Hanaki N, Kikutsuji T, Miyauchi T, Kashiwagi Y, et al. Postoperative metabolic and circulatory responses in patients that express SIRS after major digestive surgery. Hepatogastroenterology 2006;53(68):228-33. PubMed
- 26. Nishioka M, Ishikawa M, Hanaki N, Kashiwagi Y, Miki H, Miyake H, et al. Perioperative hemodynamic study of patients undergoing abdominal surgery using pulse dye densitometry. Hepatogastroenterology 2006;53(72):874-8. PubMed
References
Figure & Data
REFERENCES
Citations

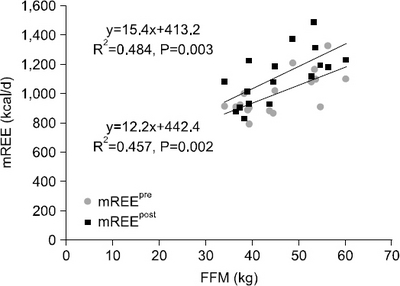
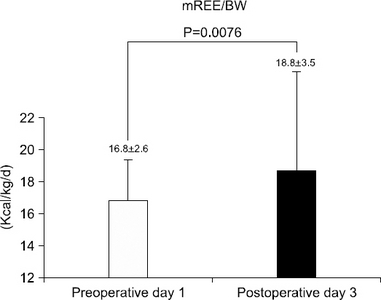
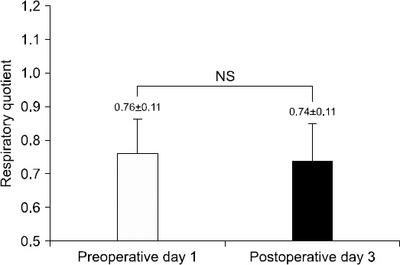
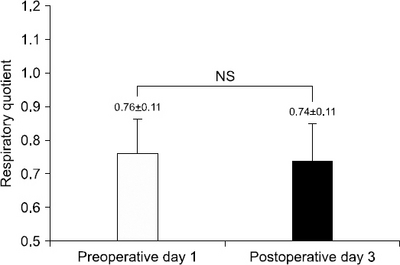
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Demographic and anthropometric data (n=17)
| Measurements | Data |
|---|---|
| Sex (male/female) | 11/6 |
| Age (y) | 62.2±12.8 |
| Weight (kg) | 60.1±8.7 |
| Height (cm) | 160.5±7.5 |
| Ideal body weight (kg) |
56.5±5.6 |
| Fat free mass (kg) | 44.2±8.7 |
| BMI (kg/m2) | 23.2±2.5 |
| Underweight |
1 (5.9) |
| Adequate | 5 (29.4) |
| Overweight | 6 (35.3) |
| Obese | 5 (29.4) |
| Diagnosis | |
| Pancreatic head carcinoma | 5 (29.4) |
| Ampulla of Vater carcinoma | 5 (29.4) |
| Distal common bile duct carcinoma | 7 (41.2) |
| Operation procedure | |
| Pylorus preserving pancreaticoduodenectomy | 11 (64.7) |
| Whipple’s procedure | 6 (35.3) |
| Nutritional status | |
| Well-nourished | 14 (82.4) |
| Moderate malnutrition | 3 (17.6) |
| Severe malnutrition | 0 (0) |
Values are presented as number only, mean±standard deviation, or number (%).
aIdeal body weight was calculated by squared meter height multiply with 22 in male (22×m2), 21 in female (21×m2).
bClassified according to the criteria of the World Health Organization Asia-Pacific, body mass index (BMI)<18.5 kg/m2 is underweight, BMI 18.5∼22.9 kg/m2 is adequate, BMI 23.0∼24.9 kg/m2 is overweight, BMI>25.0 kg/m2 is obese.
Nutrition route and energy intake (n=17)
| Nutrition route | Preoperative day 1 | Postoperative day 3 |
|---|---|---|
| Only parenteral | 12 | 0 |
| Parenteral+oral | 0 | 14 |
| NPO | 5 | 3 |
| Energy intake (kcal/kg/d) |
14.5±4.7 | 17.4±5.3 |
NPO = nil per os (nothing by mouth and no parenteral nutrition).
aMean energy intake of subjects with provided nutritional support.
Values are presented as number only, mean±standard deviation, or number (%). Ideal body weight was calculated by squared meter height multiply with 22 in male (22×m2), 21 in female (21×m2). Classified according to the criteria of the World Health Organization Asia-Pacific, body mass index (BMI)<18.5 kg/m2 is underweight, BMI 18.5∼22.9 kg/m2 is adequate, BMI 23.0∼24.9 kg/m2 is overweight, BMI>25.0 kg/m2 is obese.
NPO = nil per os (nothing by mouth and no parenteral nutrition). Mean energy intake of subjects with provided nutritional support.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

