Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > J Clin Nutr > Volume 12(2); 2020 > Article
- Original Article Effect of Nutritional Intervention by the Nutrition Support Team on Postnatal Growth in Preterm Infants
-
So Jin Yoon1,2
 , Joo Hee Lim1,2
, Joo Hee Lim1,2 , Soon Min Lee1,2
, Soon Min Lee1,2 , Sun Jung Kim3
, Sun Jung Kim3 , Sun Kyung Lee4
, Sun Kyung Lee4 , Soo Min Lee4
, Soo Min Lee4
- 미숙아에서 영양지원팀에 의한 영양중재가 출생 후 성장에 미치는 영향
-
윤소진1,2
 , 임주희1,2
, 임주희1,2 , 이순민1,2
, 이순민1,2 , 김선정3
, 김선정3 , 이선경4
, 이선경4 , 이수민4
, 이수민4
-
Journal of Clinical Nutrition 2020;12(2):26-33.
DOI: https://doi.org/10.15747/jcn.2020.12.2.26
Published online: December 31, 2020
1Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea
2Department of Pediatrics, Gangnam Severance Hospital, Seoul, Korea
3Department of Nutrition, Gangnam Severance Hospital, Seoul, Korea
4Department of Pharmacy, Gangnam Severance Hospital, Seoul, Korea
- Correspondence to Soon Min Lee https://orcid.org/0000-0003-0174-1065 Department of Pediatrics, Yonsei University College of Medicine, Gangnam Severance Hospital, 211 Eonjuro, Gangnamgu, Seoul 06273, Korea Tel: +82-2-2019-3350, Fax: +82-2-2019-4881, E-mail: smlee@yuhs.ac
© 2020, The Korean Society for Parenteral and Enteral Nutrition. All Rights Reserved.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,147 Views
- 1 Download
- 1 Crossref
Abstract
-
Purpose Nutritional intervention by an interdisciplinary nutrition support team (NST) can potentially improve postnatal growth outcomes in preterm infants. This study aimed to measure the growth impact of a nutritional intervention package performed by an NST in a quality improvement effort in a neonatal intensive care unit (NICU).
-
Methods Fifty-two infants born below 2,000 g and admitted to NICU participated in the Quality Improvement (QI) program between March 2016 and February 2017. The nutritional intervention was applied according to newly established nutritional guidelines on parenteral and enteral nutrition, and an NST performed a weekly nutritional assessment. The Z-scores of weight, height, and head circumference were calculated according to the gestational age and sex. The clinical impact on postnatal growth was compared between the QI and pre-QI groups. The pre-QI group included 69 infants admitted in the same NICU between 2014 and 2015.
-
Results The time to the initiation of enteral nutrition decreased significantly (P<0.001). Changes in weight (P=0.027), head circumference (P=0.003), Z-scores between birth, and 40 weeks postconceptional age (PCA) were significantly larger in the QI than the pre-QI group. The percentage of infants weighing below the 10th percentile at one month after birth and at 40 weeks PCA was higher in the pre-QI than the QI group.
-
Conclusion The implementation of evidence-based best practices for preterm nutrition resulted in significant improvements in the growth outcomes in preterm infants.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
ACKNOWLEDGMENTS
Conflict of interest: None.
AUTHOR CONTRIBUTIONS
Investigation: Yoon SJ
Methodology: Yoon SJ, Lim JH, Lee Soon Min
Validation: Kim SJ, Lee Soo Min
Conceptualization: Lee Soon Min, Kim SJ, Lee Soo Min
Supervision: Lee Soon Min
Software: Yoon SJ, Lee Soon Min
Writing - original draft: Yoon SJ, Lee Soon Min
Writing - review & editing: Lee Soon Min
| Variable | Pre-QI group (N=69) | QI group (N=52) | P-value |
|---|---|---|---|
| Gestational age (wk) | 30.8±2.7 | 31.1±3.0 | 0.624 |
| Birth weight (g) | 1,457±382 | 1,528±340 | 0.293 |
| Male, n (%) | 37 (53.6) | 26 (50.0) | 0.693 |
| Cesarean section | 50 (72.5) | 45 (86.5) | 0.062 |
| SGA | 4 (5.8) | 10 (19.2) | 0.041* |
| PROM | 16 (23.5) | 9 (17.3) | 0.406 |
| Chorioamnionitis | 6 (12.2) | 10 (19.2) | 0.599 |
| PIH | 4 (5.8) | 7 (13.5) | 0.203 |
| Maternal DM | 3 (4.3) | 7 (13.5) | 0.097 |
| Prenatal steroid | 19 (35.8) | 14 (26.9) | 0.513 |
| Apgar score 1 min | 4.6±1.8 | 5.0±1.3 | 0.127 |
| Apgar score 5 min | 6.7±1.4 | 6.8±1.2 | 0.686 |
| RDS | 54 (78.3) | 36 (69.2) | 0.260 |
| PDA | 25 (36.2) | 8 (14.5) | 0.007 |
| BPD | 23 (56.1) | 18 (43.9) | 0.943 |
| NEC | 0 | 0 | - |
| Cholestasis | 3 (4.3) | 7 (12.7) | 0.107 |
| Rickets | 7 (10.1) | 6 (10.9) | 0.890 |
| IVH | 11 (15.9) | 2 (3.6) | 0.037 |
| PVL | 8 (11.6) | 0 (0) | 0.009 |
| ROP | 13 (18.8) | 11 (20.0) | 0.871 |
| Sepsis | 5 (7.2) | 1 (1.8) | 0.226 |
| Hospital stay (d) | 52.0±47.4 | 51.5±42.6 | 0.950 |
Values are presented as mean±standard deviation or number (%).
- = not available; QI = quality improvement; SGA = small for gestational age; PROM = premature rupture of amniotic membrane; PIH = pregnancy-induced hypertension; DM = diabetes mellitus; RDS = respiratory distress syndrome; PDA = patent ductus arteriosus; BPD = bronchopulmonary dysplasia; IVH = intraventricular hemorrhage greater or equal to grade 3; PVL = periventricular leukomalacia; NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity.
*P-value<0.05.
- 1. Park JS, Han J, Shin JE, Lee SM, Eun HS, Park MS, et al. 2017;Postdischarge growth assessment in very low birth weight infants. Korean J Pediatr 60(3):64-9. ArticlePubMedPMCPDF
- 2. Embleton NE, Pang N, Cooke RJ. 2001;Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 107(2):270-3. ArticlePubMedPDF
- 3. Clark RH, Thomas P, Peabody J. 2003;Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 111(5 Pt 1):986-90. ArticlePubMedPDF
- 4. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. 2006;Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117(4):1253-61. ArticlePubMedPDF
- 5. Stefanescu BM, Gillam-Krakauer M, Stefanescu AR, Markham M, Kosinski JL. 2016;Very low birth weight infant care: adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum Dev 94:25-30. ArticlePubMed
- 6. Choi AY, Lee YW, Chang MY. 2016;Modification of nutrition strategy for improvement of postnatal growth in very low birth weight infants. Korean J Pediatr 59(4):165-73. ArticlePubMedPMC
- 7. Klingenberg C, Muraas FK, Isaksen CE, Nilsen T, Torgersen M, Melum-Hansen C. 2019;Growth and neurodevelopment in very preterm infants receiving a high enteral volume-feeding regimen - a population-based cohort study. J Matern Fetal Neonatal Med 32(10):1664-72. ArticlePubMed
- 8. Fenton TR, Kim JH. 2013;A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59.ArticlePubMedPMCPDF
- 9. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. 2005;Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 116(6):1353-60. ArticlePubMedPDF
- 10. Papile LA, Burstein J, Burstein R, Koffler H. 1978;Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92(4):529-34. ArticlePubMed
- 11. Walsh MC, Kliegman RM. 1986;Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33(1):179-201. ArticlePubMedPMC
- 12. Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. 2011;Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127(5):817-26. PubMedPMC
- 13. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. 2002;Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110(2 Pt 1):285-91. ArticlePubMedPDF
- 14. Hu F, Tang Q, Wang Y, Wu J, Ruan H, Lu L, et al. 2019;Analysis of nutrition support in very low-birth-weight infants with extrauterine growth restriction. Nutr Clin Pract 34(3):436-43. ArticlePubMedPDF
- 15. Shan HM, Cai W, Cao Y, Fang BH, Feng Y. 2009;Extrauterine growth retardation in premature infants in Shanghai: a multicenter retrospective review. Eur J Pediatr 168(9):1055-9. ArticlePubMedPDF
- 16. Sneve J, Kattelmann K, Ren C, Stevens DC. 2008;Implementation of a multidisciplinary team that includes a registered dietitian in a neonatal intensive care unit improved nutrition outcomes. Nutr Clin Pract 23(6):630-4. ArticlePubMedPDF
- 17. Jeong E, Jung YH, Shin SH, Kim MJ, Bae HJ, Cho YS, et al. 2016;The successful accomplishment of nutritional and clinical outcomes via the implementation of a multidisciplinary nutrition support team in the neonatal intensive care unit. BMC Pediatr 16:113.ArticlePubMedPMCPDF
- 18. Park JA, Park JE, Jeong MJ, Kim JS, Son ES, Eun HS. 2017;Influence of fish oil-containing lipid emulsions on parenteral nutrition- associated liver disease in neonates. J Clin Nutr 9(1):21-9. Article
- 19. Stevens TP, Shields E, Campbell D, Combs A, Horgan M, La Gamma EF, et al. 2018;Statewide initiative to reduce postnatal growth restriction among infants <31 weeks of gestation. J Pediatr 197:82-9. e2. PubMed
- 20. Barker DJ. 2006;Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49(2):270-83. ArticlePubMed
- 21. Pampanini V, Boiani A, De Marchis C, Giacomozzi C, Navas R, Agostino R, et al. 2015;Preterm infants with severe extrauterine growth retardation (EUGR) are at high risk of growth impairment during childhood. Eur J Pediatr 174(1):33-41. ArticlePubMedPDF
- 22. Lee BS. 2015;Nutritional strategy of early amino acid administration in very low birth weight infants. Korean J Pediatr 58(3):77-83. ArticlePubMedPMC
- 23. Dinerstein A, Nieto RM, Solana CL, Perez GP, Otheguy LE, Larguia AM. 2006;Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol 26(7):436-42. ArticlePubMedPDF
- 24. Belfort MB, Ramel SE. 2019;NICU diet, physical growth and nutrient accretion, and preterm infant brain development. Neoreviews 20(7):e385-96. ArticlePubMedPDF
- 25. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. 2009;Clinical guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients, 2009. JPEN J Parenter Enteral Nutr 33(3):255-9. ArticlePubMedPDF
- 26. Centers for Disease Control and Prevention (CDC). 2013;Notes from the field: zinc deficiency dermatitis in cholestatic extremely premature infants after a nationwide shortage of injectable zinc - Washington, DC, December 2012. MMWR Morb Mortal Wkly Rep 62(7):136-7. PubMedPMC
- 27. Gupta K, Wang H, Amin SB. 2018;Copper supplementation in premature infants with parenteral nutrition-associated cholestasis. Nutr Clin Pract 33(5):718-24. ArticlePubMedPDF
- 28. Ruktanonchai D, Lowe M, Norton SA, Garret T, Soghier L, Weiss E, et al. 2014;Zinc deficiency-associated dermatitis in infants during a nationwide shortage of injectable zinc - Washington, DC, and Houston, Texas, 2012-2013. MMWR Morb Mortal Wkly Rep 63(2):35-7. PubMedPMC
References
Figure & Data
REFERENCES
Citations

- Nutrition Supply and Growth Post Nutrition Support Team Activity in Neonatal Intensive Care Unit
Hye Min Ha, Yu Jin Jung, Yoo Rha Hong, So Yoon Choi
Pediatric Gastroenterology, Hepatology & Nutrition.2024; 27(5): 313. CrossRef
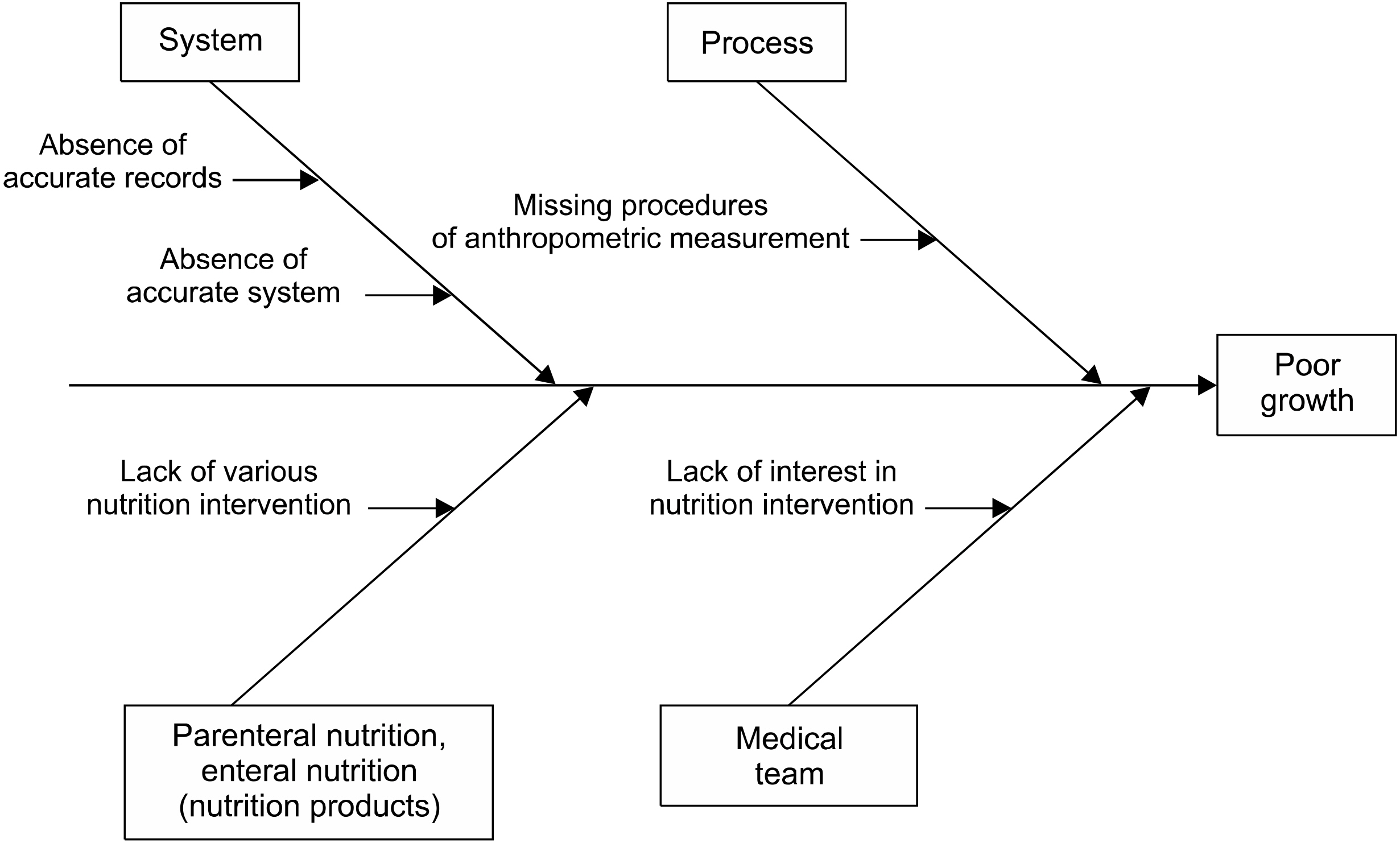
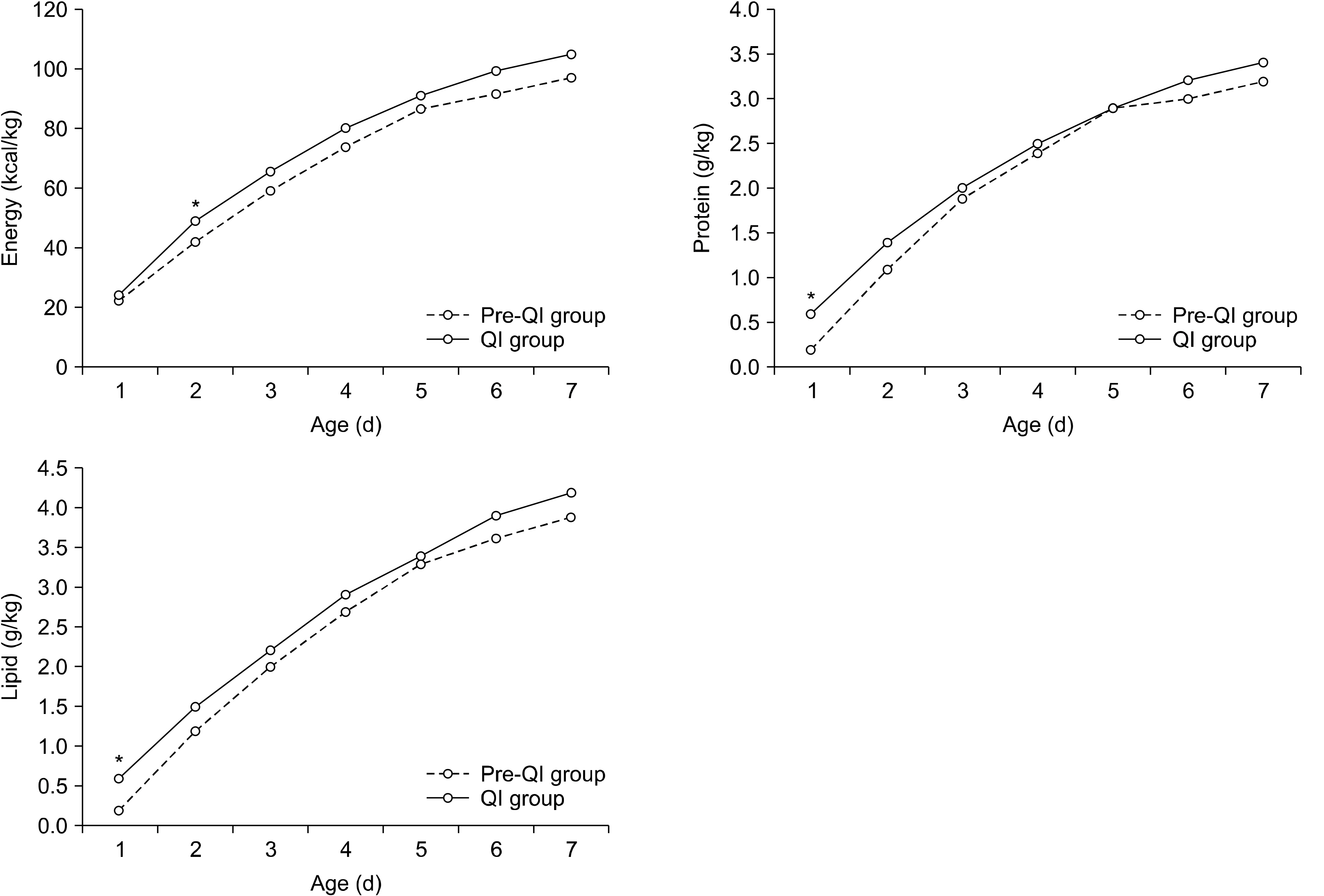
Fig. 1
Fig. 2
Patient characteristics and co-morbidities between pre-QI and QI group
| Variable | Pre-QI group (N=69) | QI group (N=52) | P-value |
|---|---|---|---|
| Gestational age (wk) | 30.8±2.7 | 31.1±3.0 | 0.624 |
| Birth weight (g) | 1,457±382 | 1,528±340 | 0.293 |
| Male, n (%) | 37 (53.6) | 26 (50.0) | 0.693 |
| Cesarean section | 50 (72.5) | 45 (86.5) | 0.062 |
| SGA | 4 (5.8) | 10 (19.2) | 0.041 |
| PROM | 16 (23.5) | 9 (17.3) | 0.406 |
| Chorioamnionitis | 6 (12.2) | 10 (19.2) | 0.599 |
| PIH | 4 (5.8) | 7 (13.5) | 0.203 |
| Maternal DM | 3 (4.3) | 7 (13.5) | 0.097 |
| Prenatal steroid | 19 (35.8) | 14 (26.9) | 0.513 |
| Apgar score 1 min | 4.6±1.8 | 5.0±1.3 | 0.127 |
| Apgar score 5 min | 6.7±1.4 | 6.8±1.2 | 0.686 |
| RDS | 54 (78.3) | 36 (69.2) | 0.260 |
| PDA | 25 (36.2) | 8 (14.5) | 0.007 |
| BPD | 23 (56.1) | 18 (43.9) | 0.943 |
| NEC | 0 | 0 | - |
| Cholestasis | 3 (4.3) | 7 (12.7) | 0.107 |
| Rickets | 7 (10.1) | 6 (10.9) | 0.890 |
| IVH | 11 (15.9) | 2 (3.6) | 0.037 |
| PVL | 8 (11.6) | 0 (0) | 0.009 |
| ROP | 13 (18.8) | 11 (20.0) | 0.871 |
| Sepsis | 5 (7.2) | 1 (1.8) | 0.226 |
| Hospital stay (d) | 52.0±47.4 | 51.5±42.6 | 0.950 |
Values are presented as mean±standard deviation or number (%).
- = not available; QI = quality improvement; SGA = small for gestational age; PROM = premature rupture of amniotic membrane; PIH = pregnancy-induced hypertension; DM = diabetes mellitus; RDS = respiratory distress syndrome; PDA = patent ductus arteriosus; BPD = bronchopulmonary dysplasia; IVH = intraventricular hemorrhage greater or equal to grade 3; PVL = periventricular leukomalacia; NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity.
*P-value<0.05.
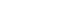
Comparisons of nutrition components during 1st week after birth between QI and pre-QI groups
| Nutrition | PND | Pre-QI group (N=78) | QI group (N=64) | P-value |
|---|---|---|---|---|
| Energy (kcal/kg) | 1 | 22.4±7.7 | 24.5±14.5 | 0.341 |
| 2 | 42.2±9.9 | 49.6±9.6 | <0.001 | |
| 3 | 59.4±11.7 | 65.9±12.0 | 0.003 | |
| 4 | 74.0±14.4 | 80.3±13.7 | 0.015 | |
| 5 | 87.0±16.1 | 91.2±15.5 | 0.147 | |
| 6 | 91.5±14.2 | 99.8±17.9 | 0.006 | |
| 7 | 97.5±16.0 | 105.3±17.0 | 0.011 | |
| Total | 473.9±69.3 | 516.6±61.1 | 0.001 | |
| Protein (g/kg) | 1 | 0.2±0.4 | 0.6±0.4 | <0.001 |
| 2 | 1.1±0.5 | 1.4±0.3 | 0.003 | |
| 3 | 1.9±0.4 | 2.0±0.5 | 0.246 | |
| 4 | 2.4±0.5 | 2.5±0.5 | 0.076 | |
| 5 | 2.9±0.5 | 2.9±0.6 | 0.708 | |
| 6 | 3.0±0.5 | 3.2±0.6 | 0.107 | |
| 7 | 3.2±0.5 | 3.4±0.5 | 0.056 | |
| Total | 14.7±2.5 | 16.0±2.4 | 0.004 | |
| Lipid (g/kg) | 1 | 0.2±0.4 | 0.6±0.5 | <0.001 |
| 2 | 1.2±0.6 | 1.5±0.4 | 0.001 | |
| 3 | 2.0±0.5 | 2.2±0.6 | 0.119 | |
| 4 | 2.7±0.7 | 2.9±0.7 | 0.057 | |
| 5 | 3.3±0.8 | 3.4±0.8 | 0.534 | |
| 6 | 3.6±0.8 | 3.9±1.0 | 0.098 | |
| 7 | 3.9±1.0 | 4.2±1.0 | 0.071 | |
| Total | 16.9±3.8 | 18.7±3.5 | 0.007 |
Values are presented as mean±standard deviation.
PND = postnatal day; QI = quality improvement.
Comparisons of nutrition and growth outcome between QI and pre-QI groups
| Nutrition and growth outcomes | Pre-QI group (N=69) | QI group (N=52) | P-value |
|---|---|---|---|
| Time to initiation of enteral feedings (d) | 3.1±2.8 | 1.4±1.6 | <0.001 |
| PN duration (d) | 26.0±29.7 | 24.2±23.1 | 0.711 |
| Time to reach full enteral feedings (d) | 20.0±16.6 | 20.7±19.2 | 0.830 |
| Full EN within 2 weeks, n (%) | 35 (50.7) | 30 (57.7) | 0.447 |
| Birth weight recovery within 2 weeks, n (%) | 50 (72.5) | 39 (75.0) | 0.754 |
| SGA at birth, n (%) | 4 (5.8) | 10 (19.2) | 0.041 |
| Weight <10th percentile at 1 month, n (%) | 25 (36.2) | 16 (30.8) | 0.530 |
| Weight <10th percentile at PCA 40 week, n (%) | 31 (44.9) | 17 (32.7) | 0.173 |
| Weight Z-score at admission | –0.18±0.81 | –0.29±1.25 | 0.603 |
| Height Z-score at admission | –0.31±0.90 | –0.66±1.47 | 0.140 |
| Head circumference Z-score at admission | –0.13±0.93 | –0.44±1.56 | 0.211 |
| Weight Z-score at 1 month | –0.98±1.04 | –0.96±0.98 | 0.904 |
| Height Z-score at 1 month | –2.02±1.55 | –1.73±1.41 | 0.301 |
| Head circumference Z-score at 1 month | –2.51±1.26 | –2.27±1.35 | 0.323 |
| Weight Z-score at PCA 40 week | –1.15±1.18 | –0.73±1.37 | 0.074 |
| Height Z-score at PCA 40 week | –0.84±1.58 | –0.75±1.48 | 0.738 |
| Head circumference Z-score at PCA 40 week | –2.25±1.07 | –1.56±1.20 | 0.005 |
| Changes of weight Z-score at PCA 40 week | –0.97±1.26 | –0.45±1.29 | 0.027 |
| Changes of weight Z-score PCA 1 month | –0.80±0.95 | –0.67±1.18 | 0.516 |
| Changes of height Z-score at PCA 40 week | –0.53±1.46 | –0.09±1.58 | 0.117 |
| Changes of head circumference Z-score at PCA 40 week | –2.06±1.26 | –1.11±1.70 | 0.003 |
Values are presented as mean±standard deviation or number (%).
QI = quality improvement; PN = parenteral nutrition; EN = enteral nutrition; PCA = post conceptional age; SGA = small for gestational age.
Values are presented as mean±standard deviation or number (%). - = not available; QI = quality improvement; SGA = small for gestational age; PROM = premature rupture of amniotic membrane; PIH = pregnancy-induced hypertension; DM = diabetes mellitus; RDS = respiratory distress syndrome; PDA = patent ductus arteriosus; BPD = bronchopulmonary dysplasia; IVH = intraventricular hemorrhage greater or equal to grade 3; PVL = periventricular leukomalacia; NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity. *P-value<0.05.
Values are presented as mean±standard deviation. PND = postnatal day; QI = quality improvement.
Values are presented as mean±standard deviation or number (%). QI = quality improvement; PN = parenteral nutrition; EN = enteral nutrition; PCA = post conceptional age; SGA = small for gestational age.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN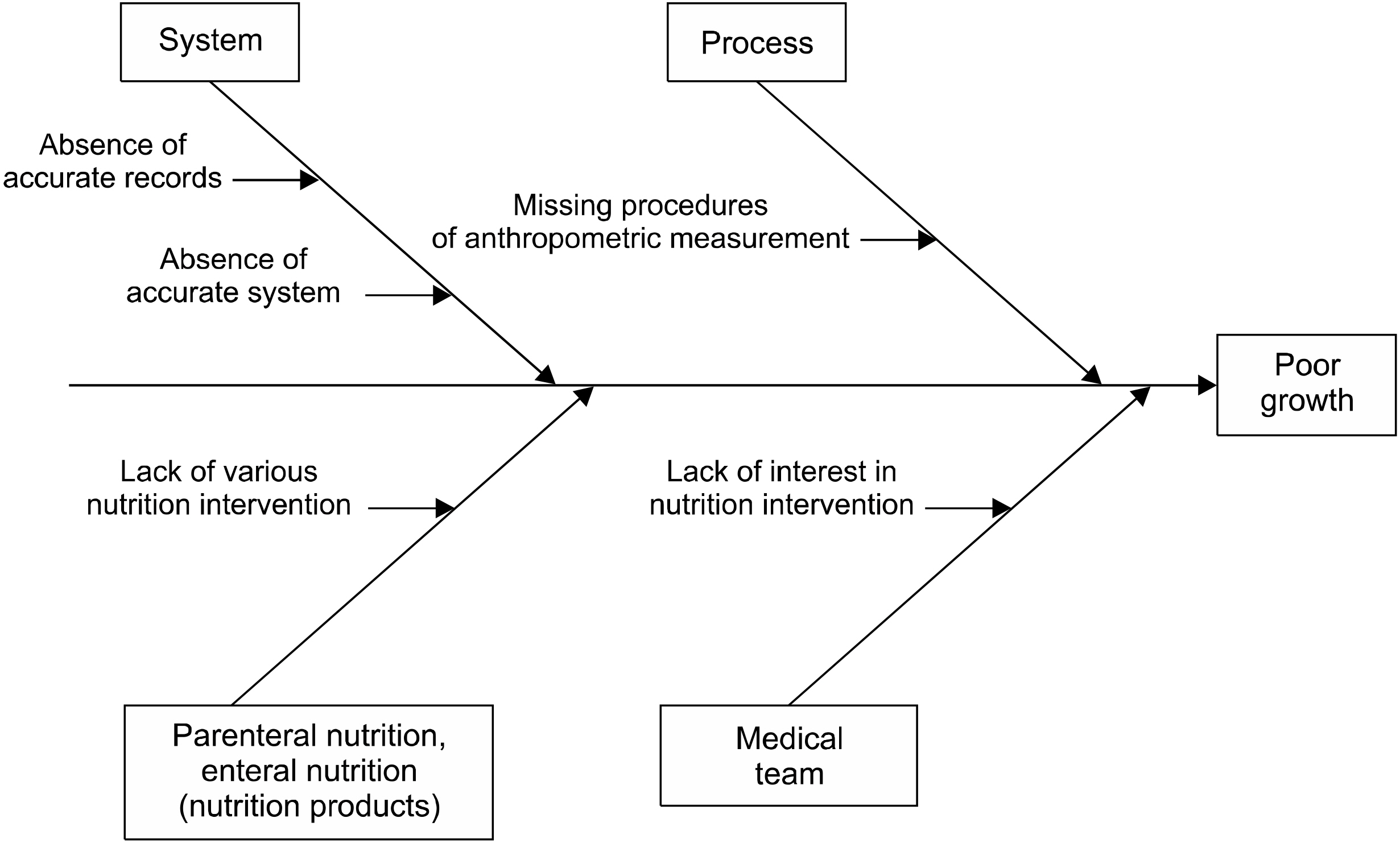
 Cite
Cite