Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 16(3); 2024 > Article
- Original article The impact of nutritional intervention by a nutrition support team on extrauterine growth restriction in very low birth weight infants in Korea: a retrospective cohort study
-
Seung Yun Lee1
 , Hye Su Hwang2
, Hye Su Hwang2 , Waonsun Im3
, Waonsun Im3 , Hyojoung Kim4
, Hyojoung Kim4 , Mi Lim Chung1
, Mi Lim Chung1
-
Annals of Clinical Nutrition and Metabolism 2024;16(3):149-157.
DOI: https://doi.org/10.15747/ACNM.2024.16.3.149
Published online: December 1, 2024
1Department of Pediatrics, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
2Department of Pediatrics, Ewha Womans University Mokdong Hospital, Seoul, Korea
3Department of Nursing, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
4Department of Pharmacy, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- Corresponding author: Mi Lim Chung, email: forevery52@naver.com
© 2024 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,313 Views
- 52 Download
Abstract
-
Purpose Achieving proper weight gain through adequate nutrition is critically important in very low birth weight (VLBW) infants. Despite recent active nutritional interventions, growth restriction is still common in VLBW infants. We aimed to determine whether nutritional intervention by a nutrition support team (NST) mitigated extrauterine growth restriction (EUGR) in VLBW infants.
-
Methods We retrospectively reviewed the medical records of VLBW infants admitted to Haeundae Paik Hospital between March 2010 and February 2024. EUGR was defined as a decrease in the weight-for-age-z-score>1.2 from birth to the postconceptional age of 36 weeks, using Fenton growth charts.
-
Results Among the 603 enrolled VLBW infants, 434 (72.0%) were diagnosed with EUGR. When comparing the control and nutritional intervention groups, the incidence of EUGR was significantly lower in infants in the intervention group (80.6% vs. 62.8%, P<0.00). Intervention group infants started enteral feeding earlier and reached half and full enteral feeding earlier (P<0.05). In addition, intravenous protein and lipid supply started sooner, increased at a faster rate, and reached peak concentrations sooner in the intervention group (P<0.05).
-
Conclusion Nutritional intervention by an NST resulted in a significant decrease in the development of EUGR in VLBW infants.
Graphical abstract
Introduction
Methods
Nutrition protocol
Results
Discussion
Acknowledgments
Authors’ contribution
Conceptualization: SYL, MLC. Data curation: HSH, SYL. Formal analysis: WI, HK. Funding acquisition: MLC. Investigation: HSH, SYL. Methodology: HSH, SYL, HK. Project administration: HSH, SYL, WI, HK. Resources: WI, HK. Software: WI, HK. Supervision: MLC. Validation: SYL, MLC, HSH. Visualization: SYL, MLC. Writing – original draft: SYL, MLC, HSH. Writing – review & editing: all authors.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
This study was supported by a 2023 grant from the Korean Society of Parenteral and Enteral Nutrition.
Data availability
Contact the corresponding author for data availability.
Supplementary materials
None.
| Variable |
Control group (N=310) |
Intervention group (N=293) |
P-value |
|---|---|---|---|
| Gestational age (wk) | 28.99±2.90 | 29.31±2.74 | 0.096a |
| Birth weight (g) | 1,104.81±264.25 | 1,140.48±278.52 | 0.051a |
| Sex, male | 150 (48.4) | 150 (51.2) | 0.491b |
| Delivery type, C-section | 253 (81.6) | 265 (90.4) | 0.002b |
| Multiple birth | 107 (34.5) | 133 (45.4) | 0.006b |
| SGA | 71 (22.9) | 68 (23.2) | 0.622c |
| RDS | 279 (90.0) | 263 (89.8) | 0.923b |
| BPD | 147 (47.4) | 135 (46.1) | 0.741b |
| Duration of invasive ventilator care (day) | 18.64±28.00 | 14.01±17.24 | 0.110a |
| Duration of non-invasive ventilator care (day) | 17.40±19.25 | 22.11±18.50 | <0.001a |
| Significant PDA | 187 (60.3) | 169 (57.7) | 0.357b |
| Significant ROP | 100 (32.3) | 50 (17.1) | <0.001b |
| NEC≥2 | 48 (15.5) | 59 (20.1) | 0.135b |
| Sepsis | 95 (30.6) | 97 (33.1) | 0.517b |
| Length of hospital stays (day) | 80.06±42.82 | 79.80±36.32 | 0.606a |
| Body weight at PCA 36 weeks (g) | 1,801.77±334.62 | 1,894.57±328.21 | 0.001a |
| EUGR at PCA 36 weeks | 250 (80.6) | 184 (62.8) | <0.000b |
Values are presented as mean±standard deviation or number (%).
SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age; EUGR = extrauterine growth restriction.
aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
| Variable |
Control group (N=310) |
Intervention group (N=293) |
P-value |
|---|---|---|---|
| Start day of enteral feeding (day) | 4.50±3.84 | 3.02±3.64 | <0.001a |
| Days until reaching half enteral feeding | 17.86±13.98 | 16.83±17.17 | 0.002a |
| Days until reaching full enteral feeding | 30.37±20.56 | 28.63±27.51 | <0.001a |
| Initial concentration of AA supply (g/kg/d) | 0.55±0.14 | 1.70±0.33 | <0.001a |
| Start day of AA supply | 1.76±0.42 | 1.00±0.00 | <0.001a |
| Day of maximum AA supply | 6.37±0.66 | 5.06±0.79 | <0.001a |
| Maximum AA concentration (g/kg/d) | 2.87±0.33 | 3.57±0.50 | <0.001a |
| Start day of lipid supply | 2.12±0.33 | 1.42±0.50 | <0.001a |
| Day of maximum lipid supply | 6.49±0.57 | 5.38±0.94 | <0.001a |
| Maximum concentration of lipid (g/kg/d) | 2.70±0.38 | 2.98±0.13 | <0.001a |
| Duration of PN (day) | 37.83±31.14 | 34.35±30.46 | 0.001a |
| PNAC | 60 (19.4) | 67 (22.9) | 0.299b |
| Variable |
Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Gestational age (wk) | 31.26±2.84 | 28.32±2.35 | <0.001a |
| Birth weight (g) | 1,264.23±250.56 | 1,066.81±259.38 | <0.001a |
| Sex, male | 87 (51.5) | 213 (49.1) | 0.596b |
| Delivery type, C-section | 151 (89.3) | 367 (84.6) | 0.129b |
| Multiple birth | 60 (35.5) | 180 (41.5) | 0.178b |
| Apgar score at 1 min | 6 (5–7) | 6 (5–6) | <0.001a |
| Apgar score at 5 min | 8 (7–8) | 7 (7–8) | <0.001a |
| SGA | 69 (40.8) | 70 (16.1) | <0.001c |
| Days of maximum initial weight loss (day) | 5.16±1.50 | 5.86±1.80 | <0.001a |
| Maximum weight loss (%) (n=578) | 9.11±4.03 | 11.20±4.66 | <0.001a |
| RDS | 127 (75.1) | 415 (95.6) | <0.001b |
| BPD | 35 (20.7) | 247 (56.9) | <0.001b |
| Significant PDA | 56 (33.1) | 300 (69.1) | <0.001b |
| Significant ROP | 13 (7.7) | 137 (31.6) | <0.001b |
| NEC≥2 | 11 (6.5) | 96 (22.1) | <0.001b |
| Sepsis | 19 (11.2) | 173 (39.9) | <0.001b |
| Body weight at PCA 36 weeks | 1,919.82±409.64 | 1,818.46±295.89 | 0.020a |
| Length of hospital stay (day) | 57.73±27.51 | 88.58±40.46 | <0.001a |
| Average weight gain (g/d) | 24.35±6.05 | 18.75±4.15 | <0.001a |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
EUGR = extrauterine growth restriction; SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age.
aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
| Variable |
Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Maternal age (yr) | 33.70±3.75 | 33.60±3.90 | 0.848a |
| Gestational diabetes | 15 (8.9) | 41 (9.4) | 0.813b |
| Pregnancy-induced hypertension | 52 (30.8) | 86 (19.8) | 0.004b |
| Premature rupture of membranes | 77 (45.6) | 232 (53.5) | 0.070b |
| Preterm labor | 94 (55.6) | 284 (65.4) | 0.019b |
| Antenatal steroid | 135 (79.9) | 365 (84.1) | 0.159b |
| Fetal distress | 66 (39.1) | 140 (32.3) | 0.129b |
| Histologic chorioamnionitis | 38 (22.5) | 143 (32.9) | 0.010b |
| Variable |
Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Days until initiation of enteral feeding | 2.34±2.21 | 4.34±4.15 | <0.001a |
| Days until reaching half enteral feeding | 10.60±8.99 | 20.00±16.81 | <0.001a |
| Days until reaching full enteral feeding | 18.64±15.46 | 33.76±25.61 | <0.001a |
| Initial concentration of AA supply (g/kg/d) | 1.27±0.59 | 1.04±0.63 | <0.001a |
| Start day of protein supply | 1.28±0.45 | 1.44±0.50 | <0.001a |
| Day of maximum protein supply | 5.44±0.95 | 5.85±0.97 | <0.001a |
| Maximum AA concentration (g/kg/d) | 3.36±0.55 | 3.15±0.54 | <0.001a |
| Start day of lipid supply | 1.57±0.53 | 1.86±0.53 | <0.001a |
| Day of maximum lipid supply | 5.63±0.95 | 6.08±0.91 | <0.001b |
| Maximum concentration of lipid (g/kg/d) | 2.88±0.27 | 2.82±0.33 | 0.051a |
| Duration of PN (day) | 22.23±18.09 | 41.57±33.02 | <0.001a |
| PNAC | 13 (7.7) | 114 (26.3) | <0.001c |
- 1. Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986-90. ArticlePubMedPDF
- 2. Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. 2015;Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000-2013. Pediatrics 136:e84-92. ArticlePubMedPDF
- 3. Lee SM, Kim N, Namgung R, Park M, Park K, Jeon J. Prediction of postnatal growth failure among very low birth weight infants. Sci Rep 2018;8:3729.ArticlePubMedPMCPDF
- 4. Stevens TP, Shields E, Campbell D, Combs A, Horgan M, La Gamma EF, et al. Statewide initiative to reduce postnatal growth restriction among infants <31 weeks of gestation. J Pediatr 2018;197:82-9.e2. ArticlePubMed
- 5. Starc M, Giangreco M, Centomo G, Travan L, Bua J. Extrauterine growth restriction in very low birth weight infants according to different growth charts: a retrospective 10 years observational study. PLoS One 2023;18:e0283367. ArticlePubMedPMC
- 6. Kim YJ, Shin SH, Cho H, Shin SH, Kim SH, Song IG, et al. 2021;Extrauterine growth restriction in extremely preterm infants based on the Intergrowth-21st Project preterm postnatal follow-up study growth charts and the Fenton growth charts. Eur J Pediatr 180:817-24. ArticlePubMedPDF
- 7. Kim ES, Sohn JA, Lee EH, Choi EJ, Lee HJ, Lee JA, et al. Extrauterine growth restriction in very low birth weight infants. J Korean Soc Neonatol 2010;17:53-63.
- 8. Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 2013;162(3 Suppl):S7-16. ArticlePubMed
- 9. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. 2006;Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117:1253-61. ArticlePubMedPDF
- 10. Weisglas-Kuperus N, Hille ET, Duivenvoorden HJ, Finken MJ, Wit JM, van Buuren S, et al. Dutch POPS-19 Collaborative Study Group. Intelligence of very preterm or very low birthweight infants in young adulthood. Arch Dis Child Fetal Neonatal Ed 2009;94:F196-200. ArticlePubMed
- 11. Sammallahti S, Pyhälä R, Lahti M, Lahti J, Pesonen AK, Heinonen K, et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr 2014;165:1109-15.e3. ArticlePubMed
- 12. Zozaya C, Díaz C, Saenz de Pipaón M. How should we define postnatal growth restriction in preterm infants? Neonatology 2018;114:177-80. ArticlePubMedPDF
- 13. Rohsiswatmo R, Kaban RK, Sjahrulla MAR, Hikmahrachim HG, Marsubrin PMT, Roeslani RD, et al. Defining postnatal growth failure among preterm infants in Indonesia. Front Nutr 2023;10:1101048. ArticlePubMedPMC
- 14. Fenton TR, Kim JH. 2013;A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59.ArticlePubMedPMCPDF
- 15. Aksoy HT, Güzoğlu N, Eras Z, Gökçe İK, Canpolat FE, Uraş N, et al. The association of early postnatal weight loss with outcome in extremely low birth weight infants. Pediatr Neonatol 2019;60:192-6. ArticlePubMed
- 16. Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, et al. Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005;147:786-90. ArticlePubMed
- 17. Arsenault D, Brenn M, Kim S, Gura K, Compher C, Simpser E, et al. American Society for Parenteral and Enteral Nutrition Board of Directors. A.S.P.E.N. Clinical Guidelines: hyperglycemia and hypoglycemia in the neonate receiving parenteral nutrition. JPEN J Parenter Enteral Nutr 2012;36:81-95. PubMed
- 18. Fallon EM, Nehra D, Potemkin AK, Gura KM, Simpser E, Compher C, et al. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support of neonatal patients at risk for necrotizing enterocolitis. JPEN J Parenter Enteral Nutr 2012;36:506-23. PubMed
- 19. Nehra D, Carlson SJ, Fallon EM, Kalish B, Potemkin AK, Gura KM, et al. American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. clinical guidelines: nutrition support of neonatal patients at risk for metabolic bone disease. JPEN J Parenter Enteral Nutr 2013;37:570-98. PubMed
- 20. Karagol BS, Zenciroglu A, Okumus N, Polin RA. Randomized controlled trial of slow vs rapid enteral feeding advancements on the clinical outcomes of preterm infants with birth weight 750-1250 g. JPEN J Parenter Enteral Nutr 2013;37:223-8. ArticlePubMedPDF
- 21. Shrikant KN, Gracy NB, Pournami F, Prithvi AK, Panackal AV, Prabhakar J, et al. Reducing extrauterine growth restriction in very preterm neonates: a before-after intervention study. Nutr Clin Pract 2024;39:1239-46. ArticlePubMed
- 22. Abiramalatha T, Thomas N, Thanigainathan S. High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database Syst Rev 2021;3:CD012413. ArticlePubMed
- 23. Repa A, Lochmann R, Unterasinger L, Weber M, Berger A, Haiden N. 2016;Aggressive nutrition in extremely low birth weight infants: impact on parenteral nutrition associated cholestasis and growth. PeerJ 4:e2483. ArticlePubMedPMCPDF
- 24. Lauriti G, Zani A, Aufieri R, Cananzi M, Chiesa PL, Eaton S, et al. 2014;Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr 38:70-85. PubMed
- 25. Lee HH, Jung JM, Nam SH, Lim G, Chung ML. Risk factor analysis of parenteral nutrition-associated cholestasis in extremely low birth weight infants. Acta Paediatr 2016;105:e313-9. ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

- Figure
- Related articles
-
- Perioperative nutritional practices and pediatric nutrition support team implementation in Korea: a cross-sectional study
- Comparison of efficacy of enteral versus parenteral nutrition in patients after esophagectomy in Malaysia: a prospective cohort study
- Consultation pattern changes of parenteral nutrition with a multidisciplinary nutrition support team in a recently opened hospital in Korea: a retrospective cohort study
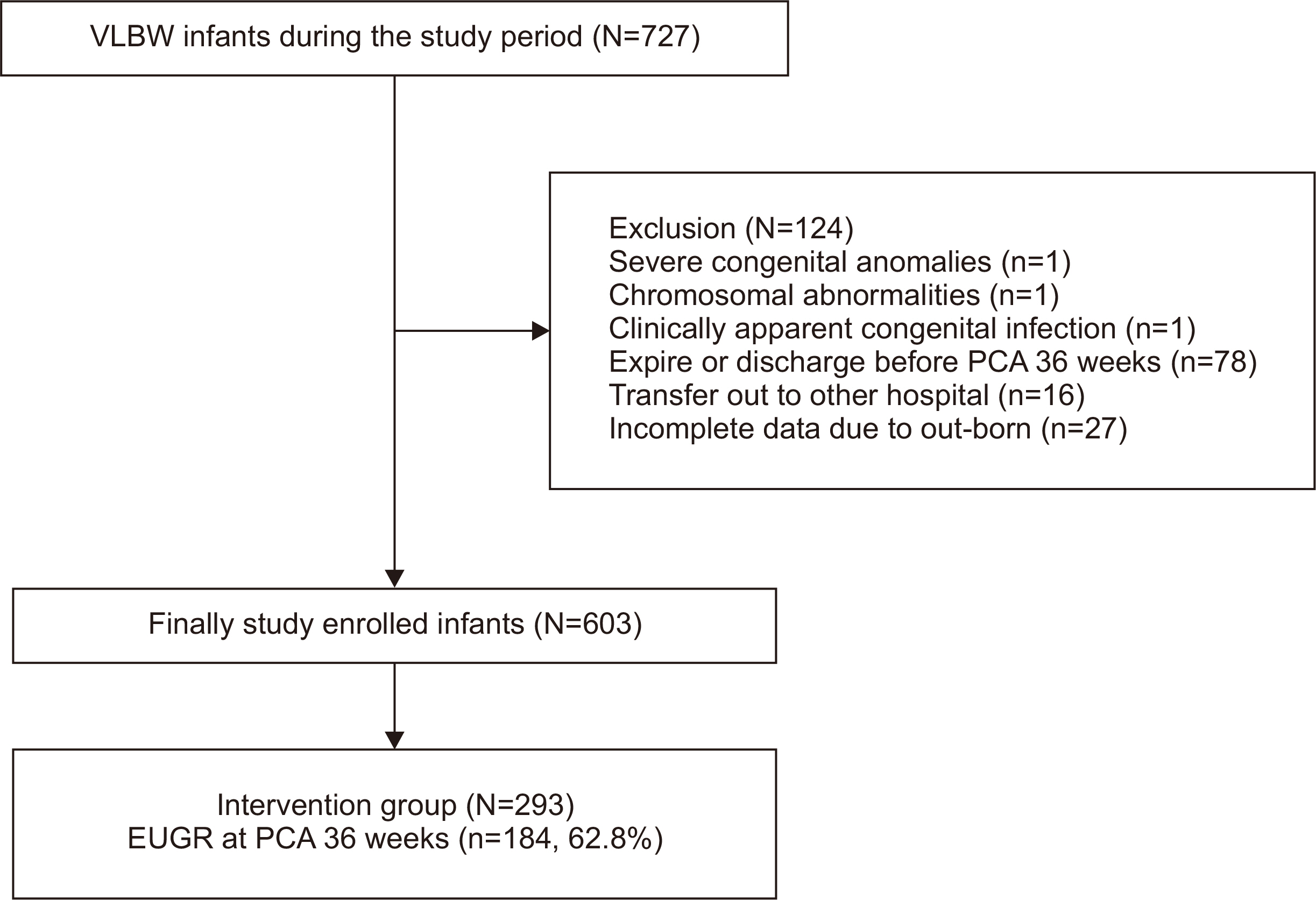
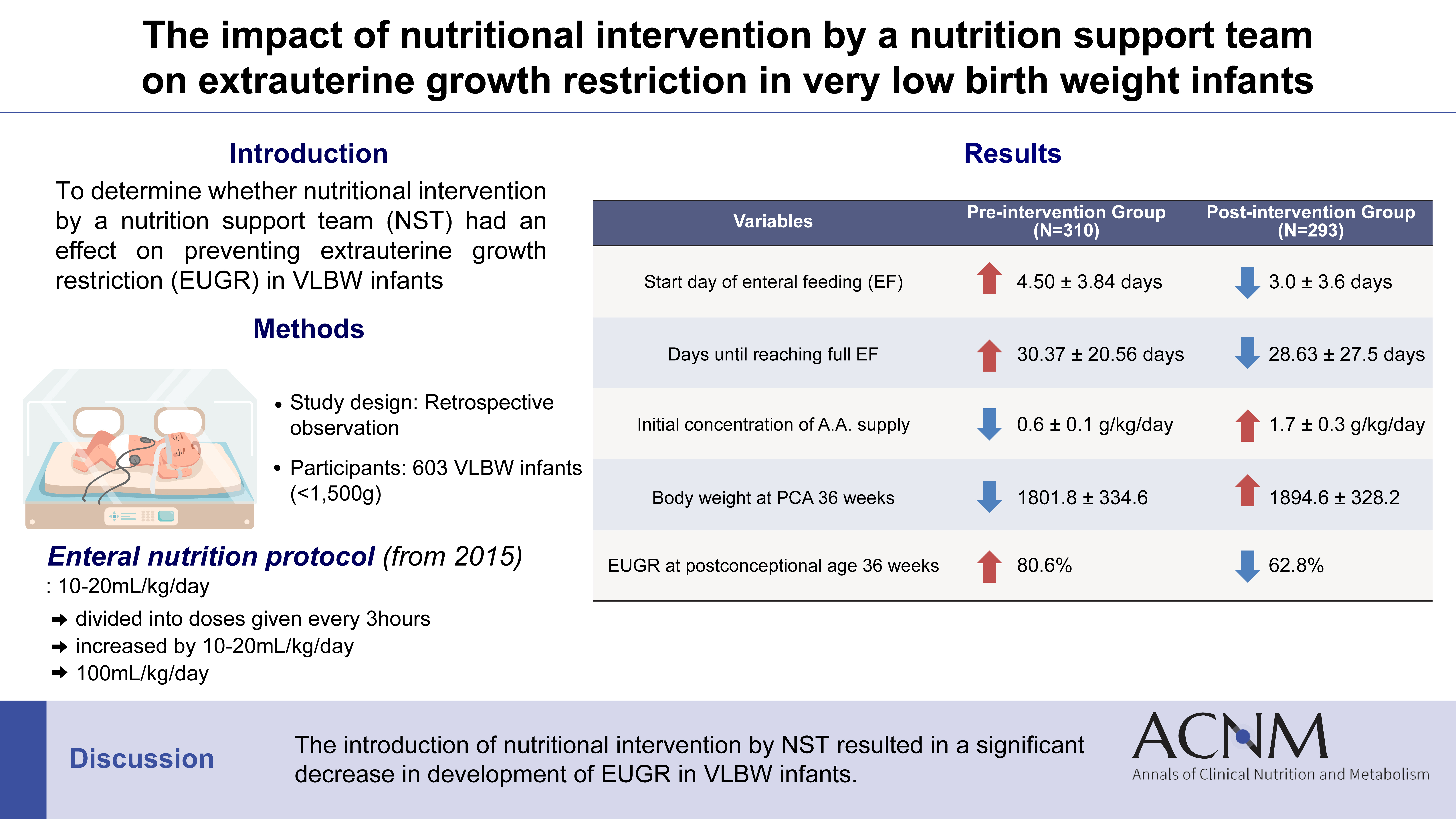
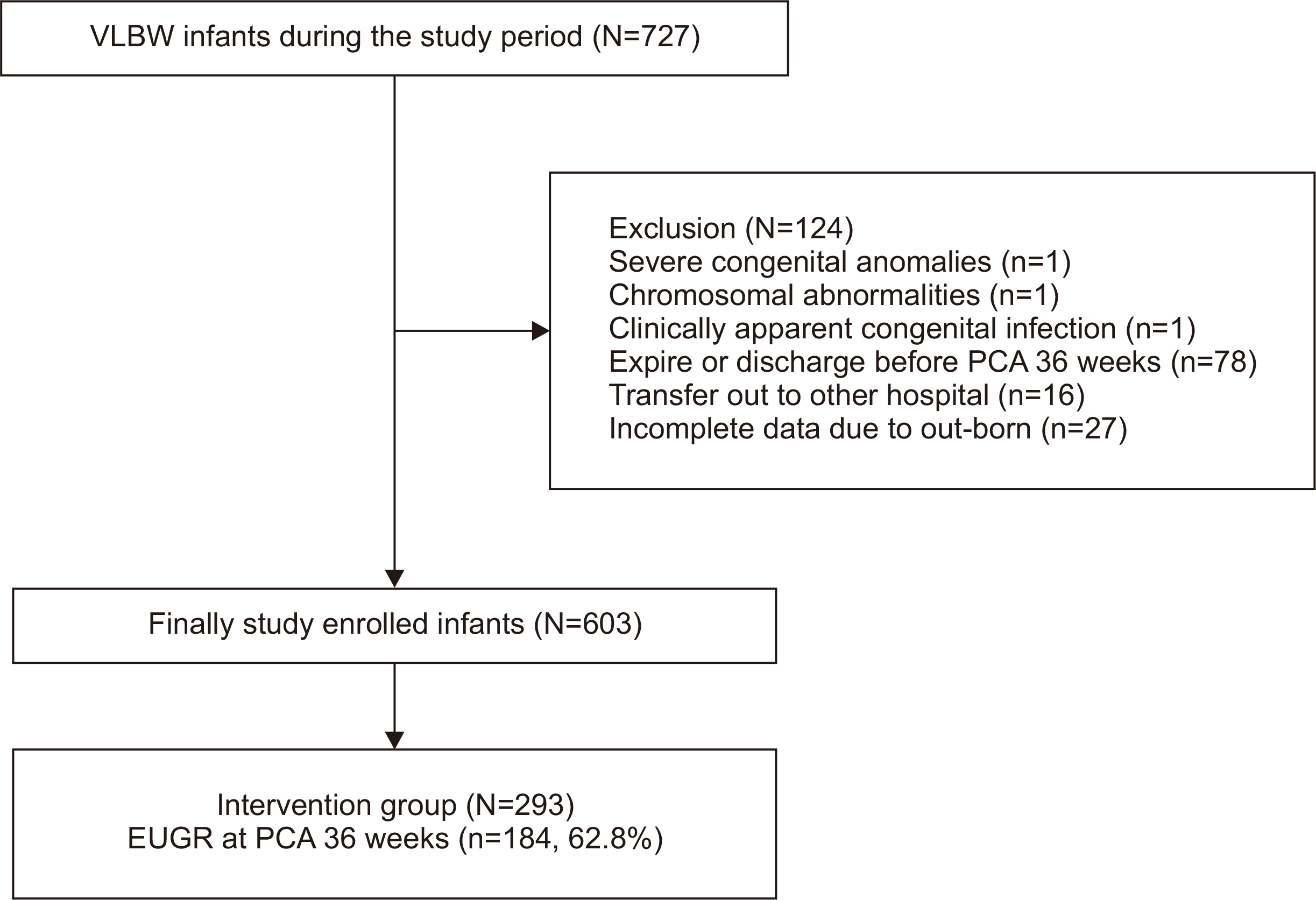
Fig. 1
Graphical abstract
Comparison between the control and intervention groups
| Variable | Control group (N=310) |
Intervention group (N=293) |
P-value |
|---|---|---|---|
| Gestational age (wk) | 28.99±2.90 | 29.31±2.74 | 0.096 |
| Birth weight (g) | 1,104.81±264.25 | 1,140.48±278.52 | 0.051 |
| Sex, male | 150 (48.4) | 150 (51.2) | 0.491 |
| Delivery type, C-section | 253 (81.6) | 265 (90.4) | 0.002 |
| Multiple birth | 107 (34.5) | 133 (45.4) | 0.006 |
| SGA | 71 (22.9) | 68 (23.2) | 0.622 |
| RDS | 279 (90.0) | 263 (89.8) | 0.923 |
| BPD | 147 (47.4) | 135 (46.1) | 0.741 |
| Duration of invasive ventilator care (day) | 18.64±28.00 | 14.01±17.24 | 0.110 |
| Duration of non-invasive ventilator care (day) | 17.40±19.25 | 22.11±18.50 | <0.001 |
| Significant PDA | 187 (60.3) | 169 (57.7) | 0.357 |
| Significant ROP | 100 (32.3) | 50 (17.1) | <0.001 |
| NEC≥2 | 48 (15.5) | 59 (20.1) | 0.135 |
| Sepsis | 95 (30.6) | 97 (33.1) | 0.517 |
| Length of hospital stays (day) | 80.06±42.82 | 79.80±36.32 | 0.606 |
| Body weight at PCA 36 weeks (g) | 1,801.77±334.62 | 1,894.57±328.21 | 0.001 |
| EUGR at PCA 36 weeks | 250 (80.6) | 184 (62.8) | <0.000 |
Values are presented as mean±standard deviation or number (%).
SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age; EUGR = extrauterine growth restriction.
aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
Nutritional parameters in the control and intervention groups
| Variable | Control group (N=310) |
Intervention group (N=293) |
P-value |
|---|---|---|---|
| Start day of enteral feeding (day) | 4.50±3.84 | 3.02±3.64 | <0.001 |
| Days until reaching half enteral feeding | 17.86±13.98 | 16.83±17.17 | 0.002 |
| Days until reaching full enteral feeding | 30.37±20.56 | 28.63±27.51 | <0.001 |
| Initial concentration of AA supply (g/kg/d) | 0.55±0.14 | 1.70±0.33 | <0.001 |
| Start day of AA supply | 1.76±0.42 | 1.00±0.00 | <0.001 |
| Day of maximum AA supply | 6.37±0.66 | 5.06±0.79 | <0.001 |
| Maximum AA concentration (g/kg/d) | 2.87±0.33 | 3.57±0.50 | <0.001 |
| Start day of lipid supply | 2.12±0.33 | 1.42±0.50 | <0.001 |
| Day of maximum lipid supply | 6.49±0.57 | 5.38±0.94 | <0.001 |
| Maximum concentration of lipid (g/kg/d) | 2.70±0.38 | 2.98±0.13 | <0.001 |
| Duration of PN (day) | 37.83±31.14 | 34.35±30.46 | 0.001 |
| PNAC | 60 (19.4) | 67 (22.9) | 0.299 |
Values are presented as mean±standard deviation or number (%).
AA = amino acid; PN = parenteral nutrition; PNAC = parenteral nutrition associated cholestasis.
aMann–Whitney U-test, bPearson’s chi square test.
Comparison between the EUGR and non-EUGR groups
| Variable | Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Gestational age (wk) | 31.26±2.84 | 28.32±2.35 | <0.001 |
| Birth weight (g) | 1,264.23±250.56 | 1,066.81±259.38 | <0.001 |
| Sex, male | 87 (51.5) | 213 (49.1) | 0.596 |
| Delivery type, C-section | 151 (89.3) | 367 (84.6) | 0.129 |
| Multiple birth | 60 (35.5) | 180 (41.5) | 0.178 |
| Apgar score at 1 min | 6 (5–7) | 6 (5–6) | <0.001 |
| Apgar score at 5 min | 8 (7–8) | 7 (7–8) | <0.001 |
| SGA | 69 (40.8) | 70 (16.1) | <0.001 |
| Days of maximum initial weight loss (day) | 5.16±1.50 | 5.86±1.80 | <0.001 |
| Maximum weight loss (%) (n=578) | 9.11±4.03 | 11.20±4.66 | <0.001 |
| RDS | 127 (75.1) | 415 (95.6) | <0.001 |
| BPD | 35 (20.7) | 247 (56.9) | <0.001 |
| Significant PDA | 56 (33.1) | 300 (69.1) | <0.001 |
| Significant ROP | 13 (7.7) | 137 (31.6) | <0.001 |
| NEC≥2 | 11 (6.5) | 96 (22.1) | <0.001 |
| Sepsis | 19 (11.2) | 173 (39.9) | <0.001 |
| Body weight at PCA 36 weeks | 1,919.82±409.64 | 1,818.46±295.89 | 0.020 |
| Length of hospital stay (day) | 57.73±27.51 | 88.58±40.46 | <0.001 |
| Average weight gain (g/d) | 24.35±6.05 | 18.75±4.15 | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
EUGR = extrauterine growth restriction; SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age.
aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
Comparison of maternal factors between the EUGR and non-EUGR groups
| Variable | Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Maternal age (yr) | 33.70±3.75 | 33.60±3.90 | 0.848 |
| Gestational diabetes | 15 (8.9) | 41 (9.4) | 0.813 |
| Pregnancy-induced hypertension | 52 (30.8) | 86 (19.8) | 0.004 |
| Premature rupture of membranes | 77 (45.6) | 232 (53.5) | 0.070 |
| Preterm labor | 94 (55.6) | 284 (65.4) | 0.019 |
| Antenatal steroid | 135 (79.9) | 365 (84.1) | 0.159 |
| Fetal distress | 66 (39.1) | 140 (32.3) | 0.129 |
| Histologic chorioamnionitis | 38 (22.5) | 143 (32.9) | 0.010 |
Values are presented as mean±standard deviation or number (%).
EUGR = extrauterine growth restriction.
aMann–Whitney U-test, bPearson’s chi square test.
Nutritional parameters in the non-EUGR and EUGR groups
| Variable | Non-EUGR group (N=169) |
EUGR group (N=434) |
P-value |
|---|---|---|---|
| Days until initiation of enteral feeding | 2.34±2.21 | 4.34±4.15 | <0.001 |
| Days until reaching half enteral feeding | 10.60±8.99 | 20.00±16.81 | <0.001 |
| Days until reaching full enteral feeding | 18.64±15.46 | 33.76±25.61 | <0.001 |
| Initial concentration of AA supply (g/kg/d) | 1.27±0.59 | 1.04±0.63 | <0.001 |
| Start day of protein supply | 1.28±0.45 | 1.44±0.50 | <0.001 |
| Day of maximum protein supply | 5.44±0.95 | 5.85±0.97 | <0.001 |
| Maximum AA concentration (g/kg/d) | 3.36±0.55 | 3.15±0.54 | <0.001 |
| Start day of lipid supply | 1.57±0.53 | 1.86±0.53 | <0.001 |
| Day of maximum lipid supply | 5.63±0.95 | 6.08±0.91 | <0.001 |
| Maximum concentration of lipid (g/kg/d) | 2.88±0.27 | 2.82±0.33 | 0.051 |
| Duration of PN (day) | 22.23±18.09 | 41.57±33.02 | <0.001 |
| PNAC | 13 (7.7) | 114 (26.3) | <0.001 |
Values are presented as mean±standard deviation or number (%).
EUGR = extrauterine growth restriction; AA = amino acid; PN = parenteral nutrition; PNAC = parenteral nutrition associated cholestasis.
aMann–Whitney U-test, bindependent t-test, cPearson’s chi square test.
Values are presented as mean±standard deviation or number (%). SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age; EUGR = extrauterine growth restriction. aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
Values are presented as mean±standard deviation or number (%). AA = amino acid; PN = parenteral nutrition; PNAC = parenteral nutrition associated cholestasis. aMann–Whitney U-test, bPearson’s chi square test.
Values are presented as mean±standard deviation, number (%), or median (interquartile range). EUGR = extrauterine growth restriction; SGA = small for gestational age; RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; PDA = patent ductus arteriosus; ROP = retinopathy of prematurity; NEC = necrotizing enterocolitis; PCA = postconceptional age. aMann–Whitney U-test, bPearson’s chi square test, cFisher’s exact test.
Values are presented as mean±standard deviation or number (%). EUGR = extrauterine growth restriction. aMann–Whitney U-test, bPearson’s chi square test.
Values are presented as mean±standard deviation or number (%). EUGR = extrauterine growth restriction; AA = amino acid; PN = parenteral nutrition; PNAC = parenteral nutrition associated cholestasis. aMann–Whitney U-test, bindependent t-test, cPearson’s chi square test.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN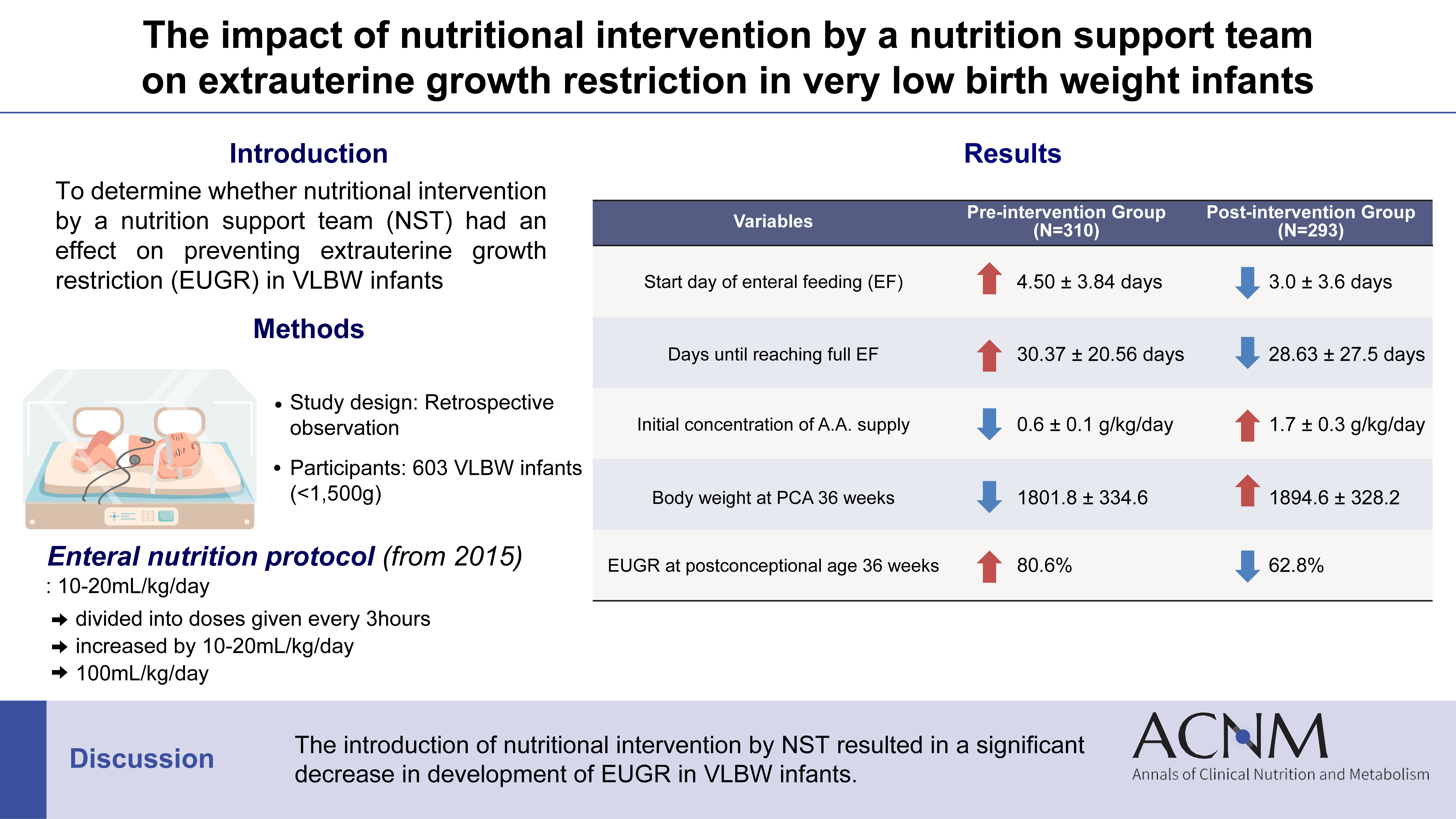
 Cite
Cite

