Abstract
-
Purpose
Parenteral nutrition (PN) is essential for the treatment of patients with malnutrition. The provision of central PN should be recommended by a nutrition support team (NST) made up of a team of experts, even in a newly established hospital. This study sought to evaluate the effectiveness of PN delivered by a multidisciplinary NST in a recently opened hospital.
-
Methods
This was a retrospective study of the effectiveness of a central PN recommendation pop-up message by the electronic medical record (EMR) software to prompt physicians to either calculate the required calorie and protein intake or consult with the NST. The study period was divided into pre-NST and post-NST based on the time of recruitment of NST-dedicated personnel.
-
Results
Patients in the 12-week pre-NST period (n=50) and 12-week post-NST period (n=74) were compared retrospectively. Baseline characteristics were not significantly different between the two groups, except for the median Acute Physiology and Chronic Health Evaluation II score (pre-NST group, 8 [interquartile range, IQR 5–15.5] vs. post-NST group, 15 [IQR 9–24], P=0.012) of the 45 patients total admitted to the intensive care unit. The percentage of patients for whom physicians requested a consultation with the NST for central PN was significantly higher in the post-NST group (52.0% vs. 75.7%, P=0.011). There was no significant difference in achievement of nutrition targets or mortality.
-
Conclusion
Building a multidisciplinary NST may increase awareness of nutritional status and affect the behavior of physicians in recently-opened hospitals.
-
Keywords: Acute Physiology and Chronic Health Evaluation (APACHE); Malnutrition; Nutritional support; Parenteral nutrition; Referral and consultation
Introduction
Background
Parenteral nutrition (PN) is crucial for patients who are unable to meet nutritional needs through oral intake [
1]. As malnutrition can have a significant impact on patient outcomes, the provision of PN is important and can be improved using a multidisciplinary approach. The nutrition support team (NST) involves healthcare professionals from various fields, including doctors, nurses, dietitians, and pharmacists [
2,
3]. Parent et al. [
4] reported that implementation of a multidisciplinary NST showed reductions in the number of patients started on PN and the total number of days that patients received PN. In the case of a newly established hospital, the implementation of a multidisciplinary NST can be challenging due to various factors, such as personnel, administration, or facilities [
5]. Nevertheless, the NST plays an essential role in hospitals to reduce metabolic complications and inappropriate PN administration [
6].
This study aimed to evaluate the effectiveness of PN delivered by a multidisciplinary NST in a recently opened hospital.
Methods
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Chung-Ang University Gwangmyeong Hospital (approval number: 2207-016-011), and the need for informed consent was waived owing to the non-interventional nature of this study.
Study design
Setting
This study was conducted in a university-affiliated, 700-bed general hospital, the Chung-Ang University Gwangmyeong Hospital. The institute newly opened in March 2022 and began operating inpatient rooms in phases. Additionally, the NST was established in April, and all members were experts with previous NST experience; however, it was not until July that all members were fully incorporated. Consequently, we divided the research period into two categories with a designated window period. The study period was from April 2022 to October 2022 and was split into the following phases: pre-NST period from April 4 to June 26 (12 weeks), a 6-week window period from June 27 to August 7, and the post-NST period from August 8 to October 28 (12 weeks).
The NST in our institute comprised 8 doctors, 3 dietitians, 2 pharmacists, and 2 nurses. One of the nurses served as the team’s coordinator, eventually becoming a member of the team. Before the full recruitment of the team (pre-NST period), the national insurance fee was unable to be claimed, and only limited consultation was conducted. The team composition was determined in July, during the window period. Furthermore, during the same period, the electronic medical record (EMR) system’s pop-up alarm was enhanced to optimize nutrition support while the team adjusted to the workflow (
Fig. 1). A refined EMR system was developed to advise physicians to either perform direct calculations of the necessary energy and protein requirements or seek consultation with the NST (
Fig. 2).
The study enrolled patients who were above 18 years of age and had received central PN. However, patients who had been hospitalized for 3 or fewer days were excluded from the research. During the study, 6 types of standardized commercial central PN products were available in our institute, and all of them were the 3-chamber type: Smofkabiven® central emulsion 1,477 mL and 986 mL (Fresenius Kabi Korea); Olimel® N9E central 1,500 mL and 1,000 mL (Baxter); and Winuf® central 1,085 mL and 736 mL (JW Life Science). None of the adult patients received individually compounded PN during the study period.
Variables
The primary outcome was the proportion of NST consultations for patients who had received central PN. The secondary outcome was the percentage of each group that achieved the nutrition targets. Energy requirement was calculated as 25–30 kcal/kg/day in critically-ill, normal weight patients and ambulatory, non-hypermetabolic patients and 30–35 kcal/kg/day in moderately and severe stressed patients. For patients with normal renal function, the recommended protein intake for maintenance was 1.0–1.2 g/kg/day, and was gradually increased to 1.5–2.0 g/kg/day for severely stressed patients. Overnutrition and undernutrition were defined as cases where the administered calorie intake exceeded or fell below the calculated requirement by 30%.
Data sources/measurement
Data on patient characteristics were obtained from the EMR by the investigators under the approval of the IRB. The registered nutritionist, registered pharmacist, and registered nurses separately reviewed the collected data. Energy intake exceeding the recommended target by 110% was defined as overfeeding [
7]. Likewise, if a patient consumed more than half of the prescribed meal while receiving a complete dose of central PN, it could be regarded as overfeeding. The following data were collected: age, sex, type of administered PN, date of admission, hospital length of stay, weight, height, body mass index, underlying disease, length of intensive care unit (ICU) stay, Acute Physiology and Chronic Health Evaluation II (APACHE II) score [
8], initial nutrition status, recommended energy and protein target, initial laboratory findings (hemoglobin, hematocrit, total lymphocyte count, albumin, and protein), in-hospital mortality, and achievement of nutrition target. Initial nutrition status was evaluated using the Global Leadership Initiative on Malnutrition (GLIM) criteria [
9]. Several complications were investigated. Hyperglycemia was defined as a blood glucose level of 200 mg/dL or higher. Liver profile elevation was defined as a significant increase in alanine aminotransferase or aspartate aminotransferase levels, specifically by 5 times or more.
There was no potential bias in selecting participating patients.
Study size
All target patients were included for the study; therefore, sample size estimation was not done.
Statistical methods
For continuous variables, the mean and standard deviation were used if the data were normally distributed, whereas the median and interquartile range (IQR) were used if the data were not normally distributed. For categorical variables, the data are presented as counts (percentages). To analyze continuous variables, either Student’s t-test or the Mann–Whitney test was used, and Pearson’s chi-squared test or Fisher’s exact test was used for categorical variables where applicable. Statistical analysis were performed using R (R Core Team [2022], R: a language and environment for statistical computing; R Foundation for Statistical Computing), and a P-value of less than 0.05 was considered statistically significant.
Results
Participants
During the study period, central PN was administered to 133 patients. However, 9 patients who had been hospitalized for less than 4 days were excluded from analysis. A total of 50 patients were included in the pre-NST period (April 4 to June 26, 2022), and 74 patients were included in the post-NST period (August 8 to October 28, 2022). The baseline characteristics of the patients in the two groups are summarized in
Table 1. No statistically significant differences were observed between the demographic characteristics of two groups. Among patients admitted to the internal medicine department, patients in the hemato-oncology subdepartment accounted for the largest proportion in both groups (20 and 41 patients in the pre-NST and post-NST groups, respectively).
In the pre-NST group, 24 patients (48.0%) were admitted to the ICU, whereas in the post-NST group, 21 patients (28.4%) were admitted to the ICU. The median ICU stay was 7 days (IQR 4–13.5 days) in the pre-NST group and 11 days (IQR 7–18 days, P=0.110) in the post-NST group. The APACHE II score showed a significant difference between the two groups, with median scores of 8 (IQR 5–15.5) in the pre-NST group and 15 (IQR 9–24) in the post-NST group (P=0.012). As a result, the predicted death rate of each group was significantly different, 8.8% (IQR 5.8–22.2) in the pre-NST group and 21.0% (IQR 12.9–49.7) in the post-NST group (P=0.005). This outcome suggests that the severity level of ICU patients may increase depending on the period.
Main results
The primary outcome, i.e., the proportion of NST consultations among patients who received central PN, was significantly increased post-NST (
Table 2). The prevalence of undernutrition was decreased; however, the achievement of nutrition targets in each group showed no statistically significant difference. This result may be attributed to increased overnutrition in the post-NST group. In particular, all seven overnutrition patients in the pre-NST group and 17 of 23 overnutrition patients in the post-NST group were admitted to the hemato-oncology subdepartment. This outcome suggests that the efforts of the NST have resulted in the provision of more appropriate nutritional recommendations; however, they may not have been adequate to induce changes in excessive nutrition support in certain subdepartments.
The other outcomes showed that initial nutrition status and laboratory findings were not significantly different between the two groups. In addition, the overall in-hospital mortality rate was similar between the two groups. Overall, there was no significant difference between the two groups (P>0.05).
Discussion
Interpretation
We investigated the impact of implementing a multidisciplinary NST in a recently opened hospital. Our findings suggest that building such a team could improve nutritional outcomes. However, we also found that maintenance could be challenging in the early stages of a hospital’s operation. The main challenge was the need to recruit and train staff from various disciplines and ensure that they worked together effectively as a team [
2]. We also found that implementing simple modifications to the hospital’s EMR alarm system could lead to significant improvements in physician behavior regarding NST consultation. However, we observed that the success of these modifications was highly dependent on the physicians’ willingness to adopt them in their daily practice.
According to the recommendations of the American Society for Parenteral and Enteral Nutrition, to ensure proper PN administration, it is important to prescribe PN that meets individual needs, monitor the patient’s response to treatment, adjust the therapeutic plan as required, and ensure a timely transition when PN is no longer needed [
1]. The NST plays an important role in ensuring proper PN administration for hospitalized patients in various situations, which could reduce complications and mortality [
6,
10-
12].
Parent et al. [
4] showed that the implementation of a weekly multidisciplinary meeting to discuss hospitalized patients receiving or being considered for PN effectively changed practice behaviors and encouraged more judicious selection of patients for PN therapy, and the results indicate that a reduction in overall PN utilization was mainly due to a decrease in short-term use. In our study, we found that in-hospital mortality was not significantly different between the two groups, even though there were more consultation requests in the post-NST group and a higher number of severe patients.
Generally, the NST has three primary roles. First, they offer guidance and consultation to healthcare professionals in hospital settings regarding PN and enteral nutrition. Second, they provide education and training to healthcare professionals. Third, they are involved in the development and implementation of nutrition-related policies [
2]. However, there is limited evidence to suggest that the NST could reduce inappropriate PN administration. Parent et al. [
4] found that their weekly multidisciplinary NST reviews led to fewer patients starting PN, fewer days spent by patients receiving PN, and fewer patients with short-term PN administration. However, in the final year of their study, there was an unexpected increase in both the number of patients starting PN and the number of patients with short-term PN administration in the ICU. After implementing the NST, we observed a similar increase in the provision of central PN in our study. Central PN is more frequently prescribed during NST consultation regardless of whether its administration is appropriate, which may explain the findings.
Moreover, central PN is mainly administered through central venous access devices (CVADs) due to its pH and high osmolality. However, using CVADs to administer PN can lead to several device-associated issues, including catheter-related bloodstream infection, catheter occlusion, and venous thrombosis. Patients who receive PN through CVADs have a higher risk of complications compared with those who use CVADs for other purposes. This is because the PN solution provides an ideal environment for microbial growth. The high concentrations of glucose, amino acids, and intralipids can promote the growth of bacteria and fungi [
13-
15]. In our study, we encountered 4 and 3 cases of CVAD infection before and after the implementation of the NST, respectively. In the post-NST group, 2 of the 3 cases of infections occurred in patients with a chemoport. Martincich et al. [
15] concluded that CVADs might be safe for the administration of PN with appropriate nursing care. However, only 4.6% of patients with an implanted venous port were included in their study population. Therefore, it is advisable to refrain from administering drugs other than anticancer medications through a chemoport in recently established hospitals until nursing competence is established.
First, the retrospective nature of the study and selection of patients may have caused some bias in the interpretation of the results. Second, our results should be carefully interpreted because the study was conducted in a single recently-opened hospital.
Generalizability
Above results can be generalized in another newly-established hospitals in Korea with same PN protocol.
Conclusion
Implementing a multidisciplinary NST in a new hospital may increase awareness regarding patient nutritional status; however, maintenance of NSTs is challenging. Simple modifications to the hospital’s EMR alarm system could lead to significant improvements in physician behavior regarding NST consultation; however, their willingness to adopt recommendations is crucial.
Supplementary materials
None.
Acknowledgments
None.
Authors’ contribution
Conceptualization: KWY, HGL, JYL, JMP. Data curation: KWY, HGL, YI, JYL, SGN. Formal analysis: KWY. Investigation: KWY, JMP, HGL, JYL. Methodology: HJK, KWY, JMP. Project administration: JYL, YI, SGN, HGL. Supervision: KWY, JMP. Writing – original draft: HJK, JMP, KWY. Writing – review & editing: all authors.
Conflict of interest
The authors of this manuscript have no conflicts of interest to declare.
Funding
None.
Data availability
The data are not publicly available due to privacy or ethical restrictions.
Fig. 1Pop-up alarm in electronic medical record system.
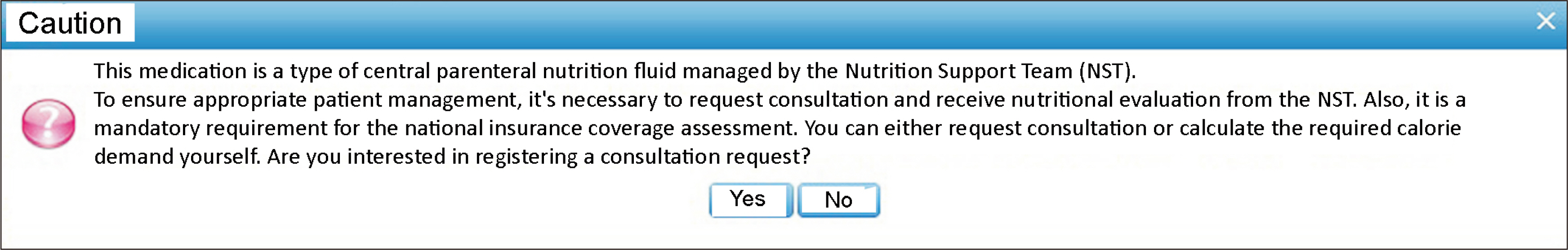
Fig. 2(A) Patient nutrition management window: physicians have the option to calculate the required calorie demand. (B) Nutrition support team consultation request window: alternatively, physicians can request appropriate nutritional support from the nutrition support team.
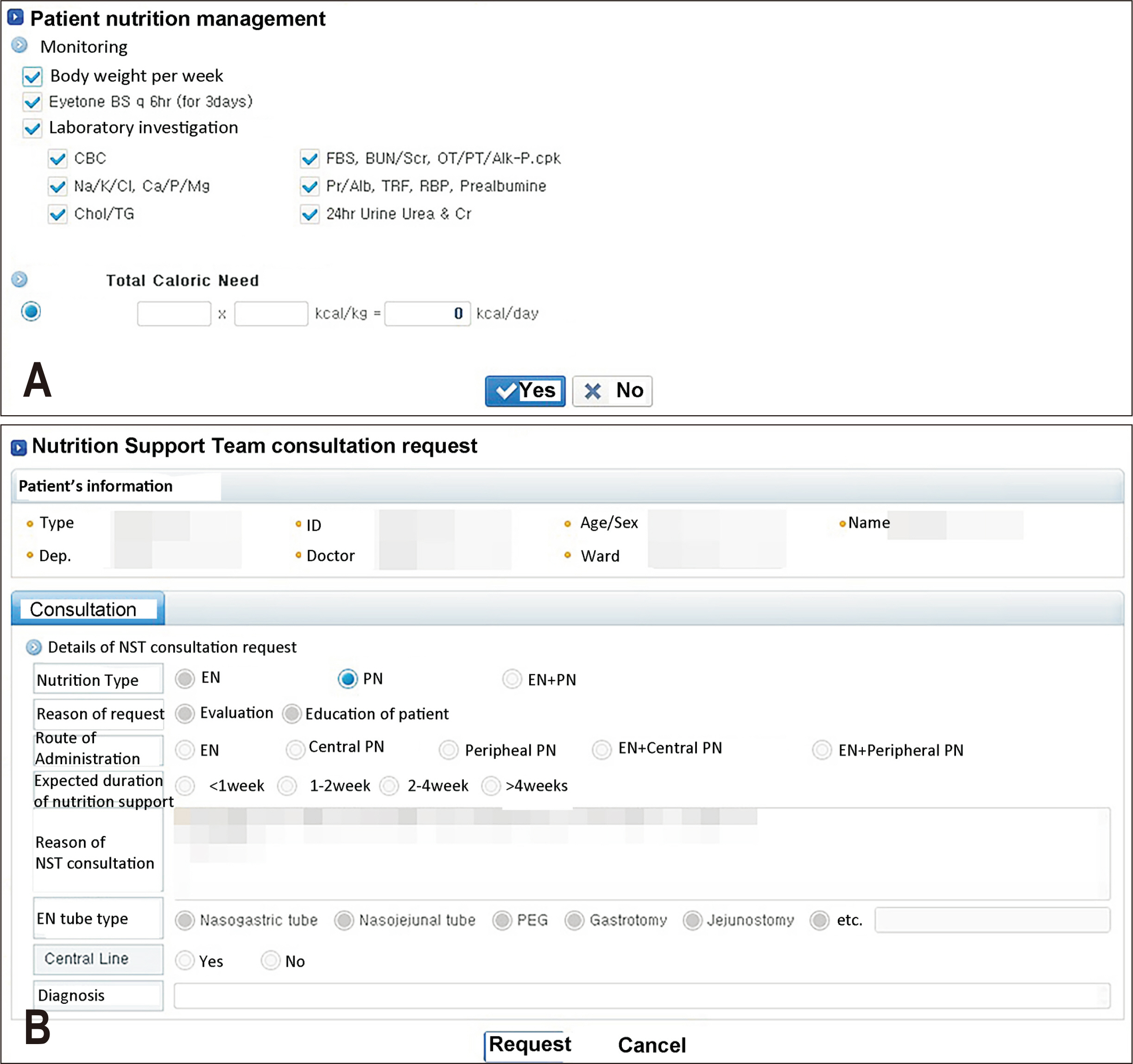
Table 1Baseline characteristics of patients
|
Characteristic |
Pre-NST (n=50) |
Post-NST (n=74) |
P-value |
|
Age (yr) |
69.1±13.2 |
66.6±13.7 |
0.304 |
|
Sex (male) |
27 (54.0) |
31 (41.9) |
0.253 |
|
Hospital length of stay (day) |
17.5 (13–26) |
18.0 (8–40) |
0.858 |
|
Weight (kg) |
53.6±11.4 |
56.6±10.0 |
0.121 |
|
Height (cm) |
160.3±8.7 |
160.0±9.1 |
0.834 |
|
Under- or overweight (kg/m2) |
|
|
0.053 |
|
Underweight (BMI<18.5) |
15 (30.0) |
13 (17.6) |
|
|
Normal range (18.5≤BMI<25.0) |
30 (60.0) |
42 (56.8) |
|
|
Overweight (25.0≤BMI) |
5 (10.0) |
19 (25.7) |
|
|
Underlying disease |
|
|
|
|
Malignancy |
32 (64.0) |
51 (68.9) |
0.706 |
|
Non-malignancy |
21 (42.0) |
36 (48.6) |
0.656 |
|
Diabetes |
16 (32.0) |
23 (31.1) |
|
|
Hypertension |
18 (36.0) |
23 (31.1) |
|
|
Coronary artery disease |
6 (12.0) |
2 (2.7) |
|
|
Chronic renal disease |
2 (4.0) |
6 (8.1) |
|
|
Others |
2 (4.0) |
3 (4.1) |
|
|
Patients who underwent operation |
18 (36.0) |
35 (47.3) |
0.332 |
|
Patients admitted to IM department |
41 (82.0) |
56 (75.7) |
0.538 |
|
Type of CVAD |
|
|
0.209 |
|
Central line |
11 (22.0) |
10 (13.5) |
|
|
PICC |
19 (38.0) |
23 (31.1) |
|
|
Chemoport |
20 (40.0) |
41 (55.4) |
|
|
Percentage of fasting patients |
34 (68.0) |
35 (47.3) |
0.036 |
|
Initial nutrition status |
|
|
0.103 |
|
Normal |
16 (32.0) |
32 (43.2) |
|
|
Mild malnutrition |
8 (16.0) |
14 (18.9) |
|
|
Moderate malnutrition |
15 (30.0) |
9 (12.2) |
|
|
Protein malnutrition |
11 (22.0) |
19 (25.7) |
|
|
Initial laboratory findings |
|
|
|
|
Hemoglobin (g/dL) |
9.5 (8.8–10.8) |
9.35 (8.2–11.1) |
0.915 |
|
Hematocrit (%) |
28.75 (25.8–32.3) |
28.50 (23.9–32.3) |
0.396 |
|
Total lymphocyte count (/µL) |
804.0 (460–1,132) |
955.5 (654–1,497) |
0.065 |
|
Albumin (g/dL) |
3.05 (2.7–3.7) |
3.2 (2.7–3.7) |
0.674 |
|
Protein (g/dL) |
5.8 (5.2–6.7) |
5.65 (5.3–6.5) |
0.693 |
Table 2
|
Characteristic |
Pre-NST (n=50) |
Post-NST (n=74) |
P-value |
|
Requested NST consultation |
26 (52.0) |
56 (75.7) |
0.011 |
|
Nutrition target |
|
|
|
|
Protein (g) |
61.5 (54.0–74.0) |
66.0 (60.0–72.0) |
0.306 |
|
Energy (kcal) |
1,500 (1,250–1,650) |
1,447 (1,320–1,650) |
0.897 |
|
Failure to achieve nutrition target |
|
|
0.165 |
|
Over-nutrition |
7 (14.0) |
23 (31.1) |
|
|
Under-nutrition |
7 (14.0) |
8 (10.8) |
|
|
Complications |
|
|
|
|
Hyperglycemia |
27 (54.0) |
35 (47.3) |
0.583 |
|
Liver profile elevation |
3 (6.0) |
3 (4.1) |
0.684 |
|
CVAD infection |
4 (8.0) |
3 (4.1) |
0.438 |
|
In-hospital mortality |
6 (12.0) |
12 (16.2) |
0.694 |
References
- 1. Worthington P, Balint J, Bechtold M, Bingham A, Chan LN, Durfee S, et al. When is parenteral nutrition appropriate? JPEN J Parenter Enteral Nutr 2017;41:324-77. ArticlePubMedPDF
- 2. Mistiaen P, Van den Heede K. Nutrition support teams: a systematic review. JPEN J Parenter Enteral Nutr 2020;44:1004-20. ArticlePubMedPDF
- 3. Guenter P, Blackmer A, Malone A, Mirtallo JM, Phillips W, Tyler R, et al. Update on use of enteral and parenteral nutrition in hospitalized patients with a diagnosis of malnutrition in the United States. Nutr Clin Pract 2022;37:94-101. ArticlePubMedPDF
- 4. Parent B, Shelton M, Nordlund M, Aarabi S, O'Keefe G. Parenteral nutrition utilization after implementation of multidisciplinary nutrition support team oversight: a prospective cohort study. JPEN J Parenter Enteral Nutr 2016;40:1151-7. ArticlePubMed
- 5. Nightingale J. Nutrition support teams: how they work, are set up and maintained. Frontline Gastroenterol 2010;1:171-7. ArticlePubMedPMC
- 6. Eriksen MK, Crooks B, Baunwall SMD, Rud CL, Lal S, Hvas CL. Systematic review with meta-analysis: effects of implementing a nutrition support team for in-hospital parenteral nutrition. Aliment Pharmacol Ther 2021;54:560-70. ArticlePubMedPMCPDF
- 7. Chapple LS, Weinel L, Ridley EJ, Jones D, Chapman MJ, Peake SL. Clinical sequelae from overfeeding in enterally fed critically ill adults: where is the evidence? JPEN J Parenter Enteral Nutr 2020;44:980-91. ArticlePubMedPDF
- 8. Capuzzo M, Valpondi V, Sgarbi A, Bortolazzi S, Pavoni V, Gilli G, et al. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med 2000;26:1779-85. ArticlePubMedPDF
- 9. Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr 2019;43:32-40. ArticlePubMed
- 10. Reber E, Strahm R, Bally L, Schuetz P, Stanga Z. Efficacy and efficiency of nutritional support teams. J Clin Med 2019;8:1281.ArticlePubMedPMC
- 11. Seol EM, Suh YS, Ju DL, Bae HJ, Kim E, Lee HJ. Nutrition support team reconsultation during nutrition therapy in Korea. JPEN J Parenter Enteral Nutr 2021;45:357-65. ArticlePubMedPDF
- 12. Oh E, Shim H, Yon HJ, Moon JS, Kang DR, Jang JY. Effectiveness of a multidisciplinary team for nutrition support in a trauma intensive care unit. Acute Crit Care 2020;35:142-8. ArticlePubMedPMCPDF
- 13. Marra AR, Opilla M, Edmond MB, Kirby DF. Epidemiology of bloodstream infections in patients receiving long-term total parenteral nutrition. J Clin Gastroenterol 2007;41:19-28. ArticlePubMed
- 14. Chopra V, O'Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2013;34:908-18. ArticlePubMed
- 15. Martincich I, Cini K, Lapkin S, Lord H, Fernandez R. Central venous access device complications in patients receiving parenteral nutrition in general ward settings: a retrospective analysis. JPEN J Parenter Enteral Nutr 2020;44:1104-11. ArticlePubMedPDF

 , Hyo Jin Kim3
, Hyo Jin Kim3 , Yujeong Im4
, Yujeong Im4 , Seul Gi Nam5
, Seul Gi Nam5 , Joo Yeon Lee6
, Joo Yeon Lee6 , Hyo Gee Lee7
, Hyo Gee Lee7 , Joong-Min Park1
, Joong-Min Park1



 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

