Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 16(3); 2024 > Article
- Original article Micronutrient deficiencies in copper, zinc, and vitamin D as predictors of clinical outcomes in critically ill surgical patients in Korea: a retrospective cohort study
-
Jiae Kim1
 , Yanghee Jun2
, Yanghee Jun2 , Ye Rim Chang2
, Ye Rim Chang2 , Jong-Kwan Baek2
, Jong-Kwan Baek2 , Hak-Jae Lee2
, Hak-Jae Lee2 , Hyewon Han1
, Hyewon Han1 , Suk-Kyung Hong2
, Suk-Kyung Hong2
-
Annals of Clinical Nutrition and Metabolism 2024;16(3):158-167.
DOI: https://doi.org/10.15747/ACNM.2024.16.3.158
Published online: December 1, 2024
1Department of Pharmacy, Asan Medical Center, Seoul, Korea
2Department of Acute Care Surgery, Asan Medical Center, Seoul, Korea
- Corresponding author: Jiae Kim, email: liberty062@gmail.com
© 2024 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,941 Views
- 66 Download
Abstract
-
Purpose To investigate the prevalence of copper, zinc, and vitamin D deficiencies in surgical intensive care unit (SICU) patients and the associations between those deficiencies and clinical outcomes.
-
Methods We conducted a retrospective study of 210 patients admitted to the SICU of Asan Medical Center between June 2020 and June 2022. Micronutrient levels were measured within 7 days of SICU admission. Primary outcomes were the mortality rate, length of SICU stay, hospital stay duration, and mechanical ventilation duration.
-
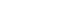
Results Copper deficiency was found in 35% (68/193), zinc deficiency in 52% (100/193), and severe vitamin D deficiency in 46% (82/179) of patients. Copper-deficient patients showed a significantly higher mortality rate (25.0% vs. 12.8%, P=0.044), longer hospital stays (57.8±47.0 vs. 45.2±36.6 days, P=0.041), and extended mechanical ventilation duration (26.9±23.3 vs. 18.8±15.7 days, P=0.012). Zinc deficiency was associated with higher C-reactive protein levels (16.2±9.5 vs. 11.5±8.8 mg/dL, P=0.001) and lower prealbumin levels (6.5±2.8 vs. 9.9±5.6 mg/dL, P<0.001). Severe vitamin D deficiency (<10 ng/mL) was not significantly associated with mortality or other clinical outcomes (mortality <10 ng/mL vs. ≥10 ng/mL, 13% vs. 18%, P=0.583).
-
Conclusion Micronutrient deficiencies are prevalent in SICU patients. Copper deficiency significantly correlated with poor clinical outcomes, and zinc deficiency showed a strong association with inflammatory markers. Early assessment and supplementation of micronutrients could be beneficial for critically ill surgical patients.
Introduction
Methods
Nutritional support protocol
Results
Discussion
Acknowledgments
Authors’ contribution
Conceptualization: JK, SKH. Data curation: YJ. Formal analysis: JK. Funding acquisition: JK. Investigation: JK, YJ. Methodology: JK. Project administration: JKB, YRC, HJL. Supervision: SKH. Writing – original draft: JK, HH. Writing – review & editing: all authors.
Conflict of interest
Suk-Kyung Hong is the Editor-in-Chief and Hak-Jae Lee is an editorial board member of the journal; however, they were not involved in the review process of this manuscript. Otherwise, there is no conflict of interest to disclose.
Funding
This research was supported by a grant from the Korean Society for Parenteral and Enteral Nutrition (KSPEN).
Data availability
Contact the corresponding author for data availability.
Supplementary materials
None.
| Characteristic |
Value (n=210) |
|---|---|
| Age (yr) | 67.9±12.6 (19–91) |
| Sex, male (%) | 64 |
| Length of hospital stay (day) (n=209) | 48.3±39.9 (8–235) |
| Length of ICU stay (day) | 23.5±22.3 (6–189) |
| ICU admission diagnosis | |
| Peritonitis | 53 (25) |
| Cancer | 79 (38) |
| AAA | 14 (7) |
| Transplantation | 11 (5) |
| Trauma | 16 (8) |
| Miscellaneousa | 51 (24) |
| Mechanical ventilation (day) | 21.1±18.6 (0–92) |
| Mortality | 34 (16) |
| 30-Day mortality | 21 (10) |
| Nutritional statusb (n=186) | |
| Well nourished | 35 (19) |
| Malnourished | 151 (81) |
Values are presented as mean±standard deviation (range) or number (%).
ICU = intensive care unit; AAA = abdominal aortic aneurysm.
aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
bASPEN (American Society for Parenteral and Enteral Nutrition).
| Micronutrient | Mean±SD level | Reference range |
Deficiency rate (%) (deficient [n]/atested [n]) |
|---|---|---|---|
| Copper (Cu) (μg/dL) | 89.5±29.8 | 76.4–145.0 | 35 (68/193) |
| Zinc (Zn) (μg/dL) | 69.1±21.7 | 66–110 | 52 (100/193) |
| 25-(OH) vitamin D (ng/mL) | 12.8±8.0 |
Deficiency<10.0 Insufficiency 10.0–30.0 Sufficiency 30.1–100.0 |
46 (82/179) |
| Copper | Low level (n=68) | Normal level (n=125) | P-value |
|---|---|---|---|
| Reference range 76.4–145.0 μg/dL | <76.4 μg/dL | 76.4–145.0 μg/dL | |
| Mortality | 17 (25.0) | 16 (12.8) | 0.044 |
| 30-Day mortality | 6 (8.8) | 13 (10.4) | 0.805 |
| ICU stay (day) | 28.3±28.3 | 21.8 ±19.6 | 0.060 |
| Hospital stay (day) | 57.8±47.0 | 45.2±36.6 | 0.041 |
| Mechanical ventilation (day) | 26.9±23.3 | 18.8±15.7 | 0.012 |
| Age (yr) | 66.1±14.4 | 69.8±11.0 | 0.071 |
| Sex, male (%) | 66 | 62 | 0.193 |
| ICU admission diagnosis | |||
| Peritonitis | 20 | 28 | 0.37 |
| Cancer | 28 | 48 | 0.82 |
| AAA | 3 | 10 | 0.55 |
| Transplantation | 2 | 9 | 0.33 |
| Trauma | 5 | 10 | >0.99 |
| Miscellaneousa | 14 | 26 | >0.99 |
| CRP (mg/dL) | 15.2±11.0 (n=58) | 13.5±8.5 (n=112) | 0.333 |
| Prealbumin (mg/dL) | 8.4±4.4 | 8.0±4.8 | 0.625 |
| Zinc (μg/dL) | 70.3±28.2 | 68.4±17.4 | 0.597 |
| Vitamin D (ng/mL) | 10.6±6.5 (n=52) | 13.6±8.1 (n=110) | 0.017 |
| Nutrition status | n=61 | n=114 | |
| Well nourished | 8 (13.1) | 23 (20.2) | 0.301 |
| Malnourished | 53 (86.9) | 91 (79.8) | 0.301 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
| Zinc | Low level (n=100) | Normal level (n=93) | P-value |
|---|---|---|---|
| Reference range 66–110 μg/dL | <66 μg/dL | 66–110 μg/dL | |
| Mortality | 13 (13) | 20 (22) | 0.129 |
| 30-Day mortality | 7 (7) | 12 (13) | 0.227 |
| ICU stay (day) | 25.78±23.7 | 22.3±22.0 | 0.289 |
| Hospital stay (day) | 50.4±37.2 | 60.5±121.6 (n=92) | 0.429 |
| Mechanical ventilation (day) | 22.8±21.5 | 20.4±16.1 | 0.379 |
| Age (yr) | 69.3±11.9 | 67.6±12.9 | 0.794 |
| Sex, male (%) | 61 | 66 | 0.191 |
| ICU admission diagnosis | |||
| Peritonitis | 19 | 29 | 0.07 |
| Cancer | 47 | 29 | 0.03 |
| AAA | 9 | 4 | 0.25 |
| Transplantation | 3 | 8 | 0.12 |
| Trauma | 6 | 9 | 0.42 |
| Miscellaneousa | 21 | 19 | >0.99 |
| CRP (mg/dL) | 16.2±9.5 (n=93) | 11.5±8.8 (n=77) | 0.001 |
| Prealbumin (mg/dL) | 6.5±2.8 | 9.9±5.6 | <0.001 |
| Copper (μg/dL) | 86.3±26.6 | 92.9±32.9 | 0.132 |
| Vitamin D (ng/mL) | 12.2±8.8 (n=83) | 13.1±6.3 (n=79) | 0.420 |
| Nutrition status | n=91 | n=84 | |
| Well nourished | 18 (20) | 13 (15) | 0.553 |
| Malnourished | 73 (80) | 71 (85) | 0.553 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
| Vitamin D | <10 (n=82) | ≥10 (n=97) | P-value |
|---|---|---|---|
| Mortality | 11 (13) | 17 (18) | 0.583 |
| 30-Day mortality | 4 (4.9) | 11 (11.3) | 0.176 |
| ICU stay (day) | 23.7±17.5 | 21.9±22.7 | 0.560 |
| Hospital stay (day) | 52.8±44.1 | 43.2±34.8 | 0.115 |
| Mechanical ventilation (day) | 23.4±20.2 | 18.4±15.4 | 0.065 |
| Age (yr) | 65.4±13.2 | 69.7±11.5 | 0.087 |
| Sex, male | 62 | 64 | 0.639 |
| ICU admission diagnosis | |||
| Peritonitis | 25 | 17 | 0.54 |
| Cancer | 36 | 32 | 0.91 |
| AAA | 5 | 8 | 0.37 |
| Transplantation | 5 | 7 | 0.54 |
| Trauma | 8 | 6 | >0.99 |
| Miscellaneousa | 22 | 18 | >0.99 |
| CRP (mg/dL) | 14.7±9.3 (n=79) | 12.9±8.3 (n=84) | 0.212 |
| Prealbumin (mg/dL) | 7.7±4.1 (n=82) | 8.5±4.7 (n=91) | 0.243 |
| Zinc (μg/dL) | 66.7±24.2 (n=75) | 71.4±18.8 (n=87) | 0.172 |
| Copper (ng/mL) | 88.2±32.5 (n=75) | 94.9±26.7 (n=87) | 0.147 |
| Nutrition status | n=72 | n=86 | |
| Well nourished | 16 (22) | 17 (20) | 0.844 |
| Malnourished | 56 (78) | 69 (80) | 0.844 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
- 1. Berger MM, Pantet O, Schneider A, Ben-Hamouda N. Micronutrient deficiencies in medical and surgical inpatients. J Clin Med 2019;8:931.ArticlePubMedPMC
- 2. Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr 2023;42:1671-89. ArticlePubMed
- 3. Agarwal A, Khanna P, Baidya DK, Arora MK. Trace elements in critical illness. J Endocrinol Metab 2011;1:57-63. Article
- 4. Btaiche IF, Chan LN, Pleva M, Kraft MD. Critical illness, gastrointestinal complications, and medication therapy during enteral feeding in critically ill adult patients. Nutr Clin Pract 2010;25:32-49. ArticlePubMedPDF
- 5. Duncan A, Talwar D, McMillan DC, Stefanowicz F, O'Reilly DS. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr 2012;95:64-71. ArticlePubMed
- 6. Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 2003;57:386-98. ArticlePubMedPMC
- 7. Yu L, Yousuf S, Yousuf S, Yeh J, Biggins SW, Morishima C, et al. Copper deficiency is an independent risk factor for mortality in patients with advanced liver disease. Hepatol Commun 2023;7:e0076. ArticlePubMedPMC
- 8. Hackler J, Heller RA, Sun Q, Schwarzer M, Diegmann J, Bachmann M, et al. 2021;Relation of serum copper status to survival in COVID-19. Nutrients 13:1898.ArticlePubMedPMC
- 9. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients 2017;9:624.ArticlePubMedPMC
- 10. Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 2013;4:176-90. ArticlePubMedPMC
- 11. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of im이대로 내도 mune function. Nutrients 2017;9:1286.ArticlePubMedPMC
- 12. Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020;12:2098.ArticlePubMedPMC
- 13. Aschner M, Erikson K. Manganese. Adv Nutr 2017;8:520-1. ArticlePubMedPMC
- 14. Lee YH, Bang ES, Lee JH, Lee JD, Kang DR, Hong J, et al. Serum concentrations of trace elements zinc, copper, selenium, and manganese in critically ill patients. Biol Trace Elem Res 2019;188:316-25. ArticlePubMedPDF
- 15. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients 2020;12:2097. ArticlePubMedPMC
- 16. Kazemi A, Mohammadi V, Aghababaee SK, Golzarand M, Clark CCT, Babajafari S. 2021;Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis. Adv Nutr 12:1636-58. ArticlePubMedPMCPDF
- 17. Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg 2012;204:37-43. ArticlePubMedPMC
- 18. Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 2014;312:1520-30. ArticlePubMed
- 19. Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, et al. ESPEN micronutrient guideline. Clin Nutr 2022;41:1357-424. ArticlePubMed
- 20. White JV, Guenter P, Jensen G, Malone A, Schofield M. Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275-83. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

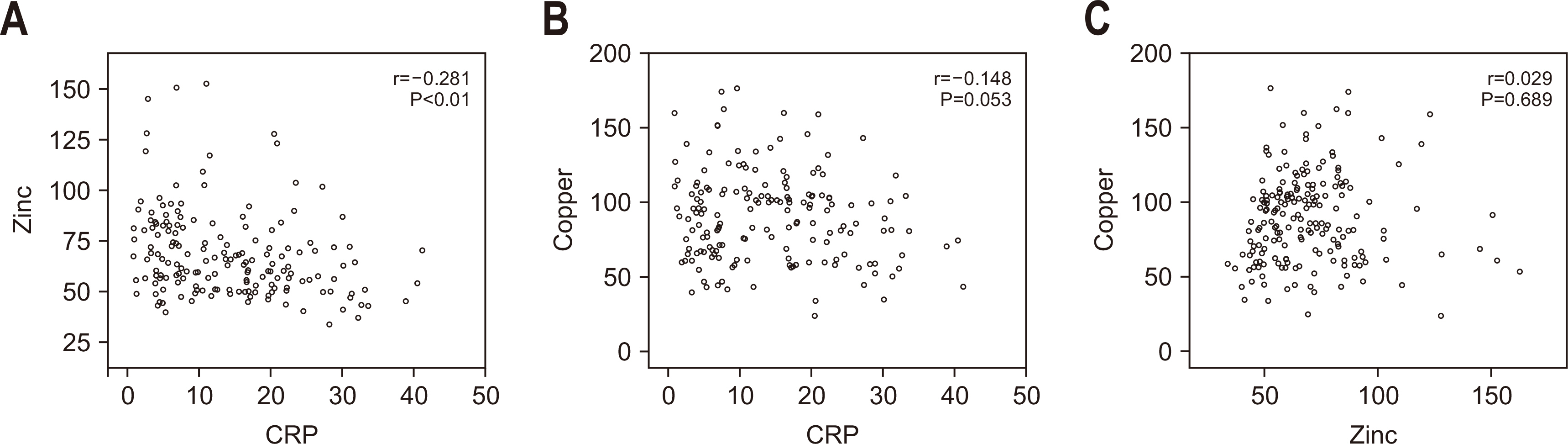
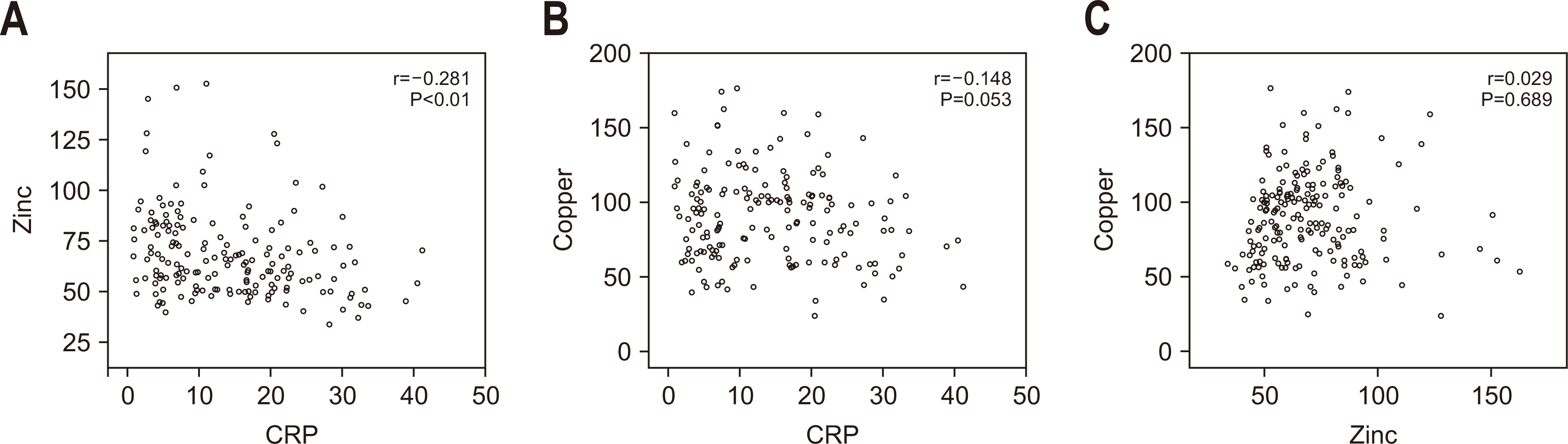
Fig. 1
Fig. 2
Baseline characteristics of the study population
| Characteristic | Value (n=210) |
|---|---|
| Age (yr) | 67.9±12.6 (19–91) |
| Sex, male (%) | 64 |
| Length of hospital stay (day) (n=209) | 48.3±39.9 (8–235) |
| Length of ICU stay (day) | 23.5±22.3 (6–189) |
| ICU admission diagnosis | |
| Peritonitis | 53 (25) |
| Cancer | 79 (38) |
| AAA | 14 (7) |
| Transplantation | 11 (5) |
| Trauma | 16 (8) |
| Miscellaneous |
51 (24) |
| Mechanical ventilation (day) | 21.1±18.6 (0–92) |
| Mortality | 34 (16) |
| 30-Day mortality | 21 (10) |
| Nutritional status |
|
| Well nourished | 35 (19) |
| Malnourished | 151 (81) |
Values are presented as mean±standard deviation (range) or number (%).
ICU = intensive care unit; AAA = abdominal aortic aneurysm.
aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
bASPEN (American Society for Parenteral and Enteral Nutrition).
Prevalence of micronutrient deficiencies in SICU patients (N=210)
| Micronutrient | Mean±SD level | Reference range | Deficiency rate (%) (deficient [n]/ |
|---|---|---|---|
| Copper (Cu) (μg/dL) | 89.5±29.8 | 76.4–145.0 | 35 (68/193) |
| Zinc (Zn) (μg/dL) | 69.1±21.7 | 66–110 | 52 (100/193) |
| 25-(OH) vitamin D (ng/mL) | 12.8±8.0 | Deficiency<10.0 Insufficiency 10.0–30.0 Sufficiency 30.1–100.0 |
46 (82/179) |
SICU = surgical intensive care unit; SD = standard deviation.
aOnly measurements performed within 7 days of ICU admission were included in the analysis.
Comparison of clinical outcomes and baseline characteristics between copper-deficient and normal groups
| Copper | Low level (n=68) | Normal level (n=125) | P-value |
|---|---|---|---|
| Reference range 76.4–145.0 μg/dL | <76.4 μg/dL | 76.4–145.0 μg/dL | |
| Mortality | 17 (25.0) | 16 (12.8) | 0.044 |
| 30-Day mortality | 6 (8.8) | 13 (10.4) | 0.805 |
| ICU stay (day) | 28.3±28.3 | 21.8 ±19.6 | 0.060 |
| Hospital stay (day) | 57.8±47.0 | 45.2±36.6 | 0.041 |
| Mechanical ventilation (day) | 26.9±23.3 | 18.8±15.7 | 0.012 |
| Age (yr) | 66.1±14.4 | 69.8±11.0 | 0.071 |
| Sex, male (%) | 66 | 62 | 0.193 |
| ICU admission diagnosis | |||
| Peritonitis | 20 | 28 | 0.37 |
| Cancer | 28 | 48 | 0.82 |
| AAA | 3 | 10 | 0.55 |
| Transplantation | 2 | 9 | 0.33 |
| Trauma | 5 | 10 | >0.99 |
| Miscellaneous |
14 | 26 | >0.99 |
| CRP (mg/dL) | 15.2±11.0 (n=58) | 13.5±8.5 (n=112) | 0.333 |
| Prealbumin (mg/dL) | 8.4±4.4 | 8.0±4.8 | 0.625 |
| Zinc (μg/dL) | 70.3±28.2 | 68.4±17.4 | 0.597 |
| Vitamin D (ng/mL) | 10.6±6.5 (n=52) | 13.6±8.1 (n=110) | 0.017 |
| Nutrition status | n=61 | n=114 | |
| Well nourished | 8 (13.1) | 23 (20.2) | 0.301 |
| Malnourished | 53 (86.9) | 91 (79.8) | 0.301 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
Comparison of clinical outcomes and baseline characteristics between zinc-deficient and normal groups
| Zinc | Low level (n=100) | Normal level (n=93) | P-value |
|---|---|---|---|
| Reference range 66–110 μg/dL | <66 μg/dL | 66–110 μg/dL | |
| Mortality | 13 (13) | 20 (22) | 0.129 |
| 30-Day mortality | 7 (7) | 12 (13) | 0.227 |
| ICU stay (day) | 25.78±23.7 | 22.3±22.0 | 0.289 |
| Hospital stay (day) | 50.4±37.2 | 60.5±121.6 (n=92) | 0.429 |
| Mechanical ventilation (day) | 22.8±21.5 | 20.4±16.1 | 0.379 |
| Age (yr) | 69.3±11.9 | 67.6±12.9 | 0.794 |
| Sex, male (%) | 61 | 66 | 0.191 |
| ICU admission diagnosis | |||
| Peritonitis | 19 | 29 | 0.07 |
| Cancer | 47 | 29 | 0.03 |
| AAA | 9 | 4 | 0.25 |
| Transplantation | 3 | 8 | 0.12 |
| Trauma | 6 | 9 | 0.42 |
| Miscellaneous |
21 | 19 | >0.99 |
| CRP (mg/dL) | 16.2±9.5 (n=93) | 11.5±8.8 (n=77) | 0.001 |
| Prealbumin (mg/dL) | 6.5±2.8 | 9.9±5.6 | <0.001 |
| Copper (μg/dL) | 86.3±26.6 | 92.9±32.9 | 0.132 |
| Vitamin D (ng/mL) | 12.2±8.8 (n=83) | 13.1±6.3 (n=79) | 0.420 |
| Nutrition status | n=91 | n=84 | |
| Well nourished | 18 (20) | 13 (15) | 0.553 |
| Malnourished | 73 (80) | 71 (85) | 0.553 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
Comparison of clinical outcomes and baseline characteristics between severe (<10 ng/mL) and non-severe (≥10 ng/mL) vitamin D deficiency groups
| Vitamin D | <10 (n=82) | ≥10 (n=97) | P-value |
|---|---|---|---|
| Mortality | 11 (13) | 17 (18) | 0.583 |
| 30-Day mortality | 4 (4.9) | 11 (11.3) | 0.176 |
| ICU stay (day) | 23.7±17.5 | 21.9±22.7 | 0.560 |
| Hospital stay (day) | 52.8±44.1 | 43.2±34.8 | 0.115 |
| Mechanical ventilation (day) | 23.4±20.2 | 18.4±15.4 | 0.065 |
| Age (yr) | 65.4±13.2 | 69.7±11.5 | 0.087 |
| Sex, male | 62 | 64 | 0.639 |
| ICU admission diagnosis | |||
| Peritonitis | 25 | 17 | 0.54 |
| Cancer | 36 | 32 | 0.91 |
| AAA | 5 | 8 | 0.37 |
| Transplantation | 5 | 7 | 0.54 |
| Trauma | 8 | 6 | >0.99 |
| Miscellaneous |
22 | 18 | >0.99 |
| CRP (mg/dL) | 14.7±9.3 (n=79) | 12.9±8.3 (n=84) | 0.212 |
| Prealbumin (mg/dL) | 7.7±4.1 (n=82) | 8.5±4.7 (n=91) | 0.243 |
| Zinc (μg/dL) | 66.7±24.2 (n=75) | 71.4±18.8 (n=87) | 0.172 |
| Copper (ng/mL) | 88.2±32.5 (n=75) | 94.9±26.7 (n=87) | 0.147 |
| Nutrition status | n=72 | n=86 | |
| Well nourished | 16 (22) | 17 (20) | 0.844 |
| Malnourished | 56 (78) | 69 (80) | 0.844 |
Values are presented as number (%), mean±standard deviation, or number only.
ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein.
aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
Values are presented as mean±standard deviation (range) or number (%). ICU = intensive care unit; AAA = abdominal aortic aneurysm. aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis. bASPEN (American Society for Parenteral and Enteral Nutrition).
SICU = surgical intensive care unit; SD = standard deviation. aOnly measurements performed within 7 days of ICU admission were included in the analysis.
Values are presented as number (%), mean±standard deviation, or number only. ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein. aPneumonia, Fournier’s gangrene, necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
Values are presented as number (%), mean±standard deviation, or number only. ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein. aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.
Values are presented as number (%), mean±standard deviation, or number only. ICU = intensive care unit; AAA = abdominal aortic aneurysm; CRP = C-reactive protein. aPneumonia, Fournier’s gangrene, Necrotizing pancreatitis, subglottic stenosis, bladder perforation, urosepsis, CPCR (cardio-pulmonary cerebral resuscitation) survivor, gallbladder perforation, cytomegalovirus esophagitis, deep neck infection, graft failure, biliary sepsis.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN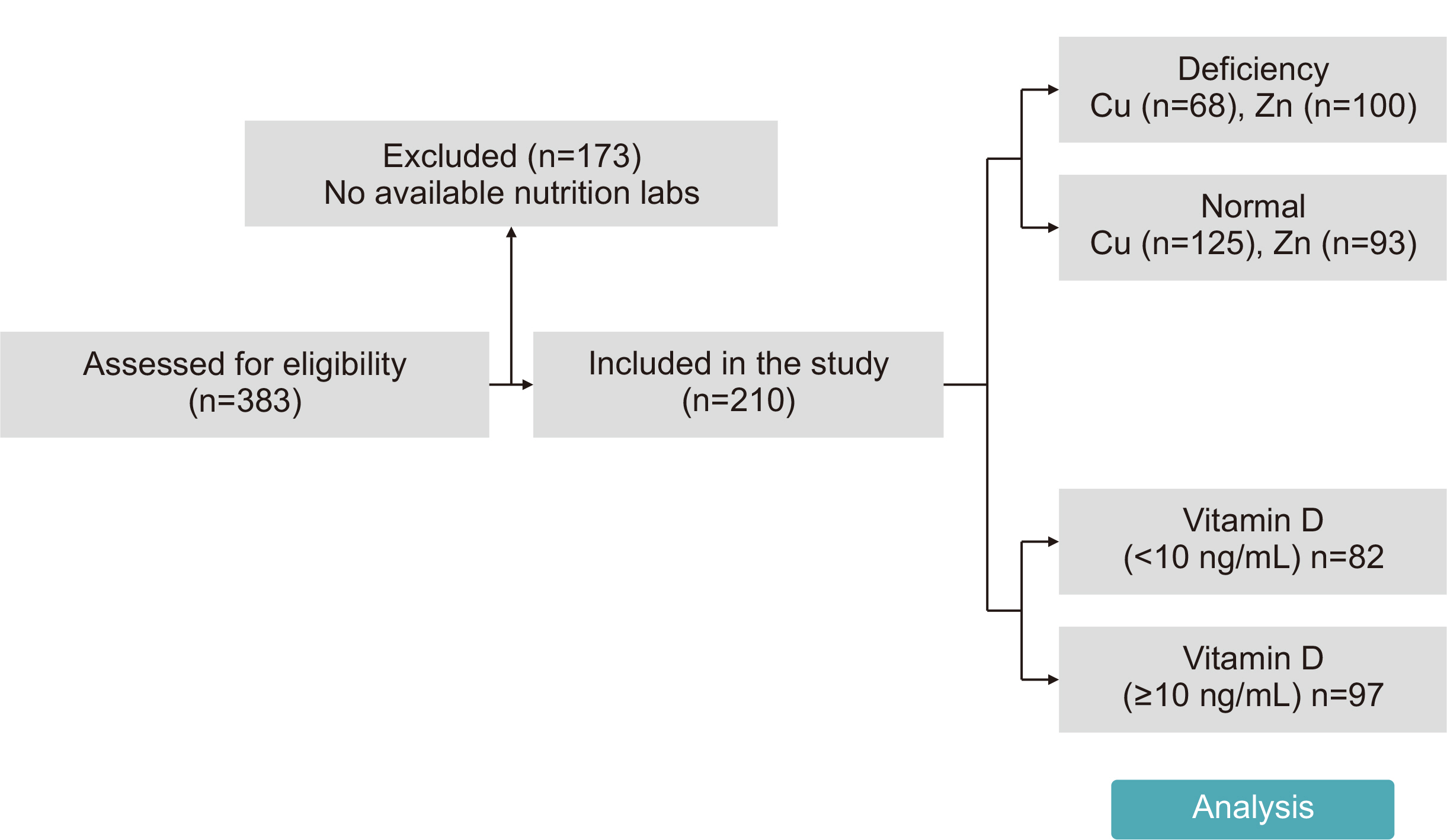
 Cite
Cite