Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 9(1); 2018 > Article
- Original Article Risk of Malnutrition after Gastrointestinal Cancer Surgery: A Propensity Score Matched Retrospective Cohort Study
- Sung-Hoon Yoon, M.D.1, Bong-Hyeon Kye, M.D., Ph.D.2, Hyung-Jin Kim, M.D.1, Kyong-Hwa Jun, M.D., Ph.D.1, Hyeon-Min Cho, M.D., Ph.D.1, Hyung-Min Chin, M.D., Ph.D.1
-
Surgical Metabolism and Nutrition 2018;9(1):16-25.
DOI: https://doi.org/10.18858/smn.2018.9.1.16
Published online: June 30, 2018
Department of Surgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Department of Surgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Correspondence to: Bong-Hyeon Kye, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpodaero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-6763, Fax: +82-2-595-2992, E-mail: ggbong@catholic.ac.kr
Copyright: © The Korean Society of Surgical Metabolism and Nutrition
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,025 Views
- 12 Download
- 3 Crossref
Abstract
-
Purpose: Patients with cancers arising from the gastrointestinal tract can suffer from nutritional inadequacies caused by various factors. This study investigated the risk of malnutrition after curative surgery in patients with gastric cancer (GC) or colorectal cancer (CRC) using various preoperative and postoperative nutritional screening tools.
-
Materials and Methods: In the authors’ hospital, 407 patients (206 patients with GC and 201 patients with CRC) underwent surgery between July 2011 and June 2012. The patients from the two groups were matched using the propensity score and then analyzed the nutritional data from 170 patients (85 patients in each group), retrospectively.
-
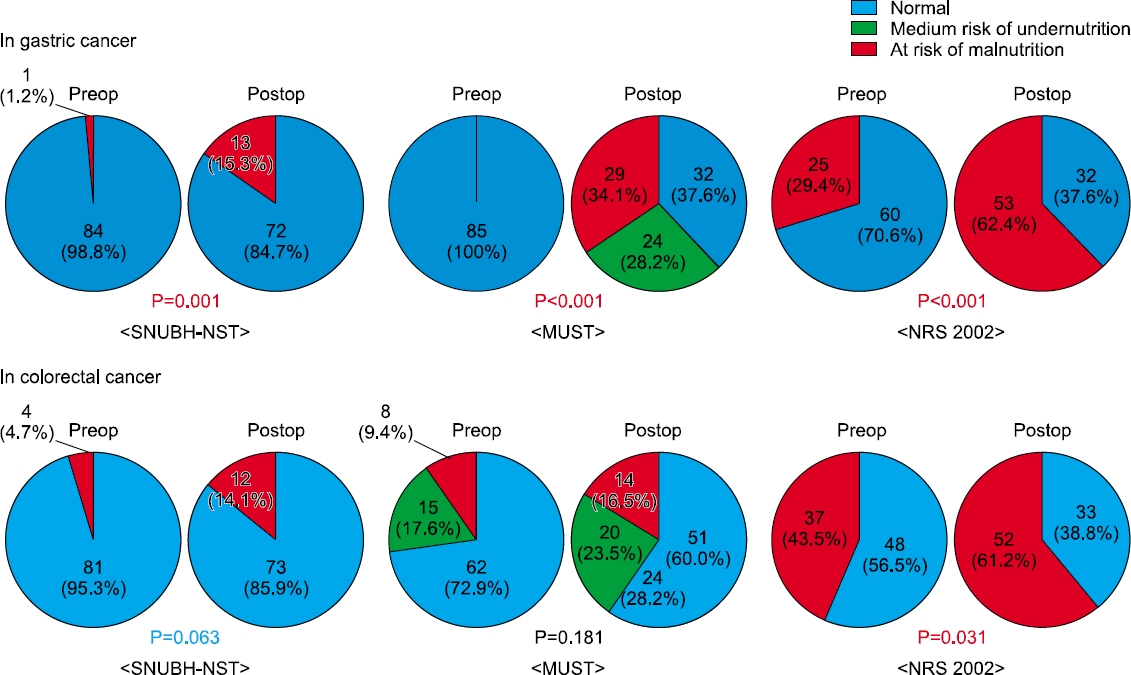
Results: In both groups, the postoperative nutritional status was impaired significantly compared to the preoperative status. The postoperative risk of undernutrition in CRC patients was significantly lower than that of the GC patients according to the Malnutrition Universal Screening Tool (P=0.007). At the time of hospital discharge after surgery, the incidence of a lower serum albumin level (P=0.002) and more than 5% weight loss (P=0.013) were higher in the GC group than in the CRC group. A comparison of the postoperative nutritional status among the types of surgery in each group, total gastrectomy in the GC group (P=0.015) and proctectomy with diverting stoma in the CRC group (P=0.06), were related to more than 5% weight loss.
-
Conclusion: Gastrointestinal cancer surgery might increase the patients’ postoperative risk of malnutrition, particularly in GC surgery. Therefore, consecutive assessments of the nutritional status and appropriate nutritional support are necessary after surgery for GC and CRC.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
| Gastric cancer (N=85) | Colorectal cancer (N=85) | P-value | ||
|---|---|---|---|---|
| Age (years) | ≤65 | 49 (57.6%) | 48 (56.5%) | |
| >65 | 36 (42.4%) | 37 (43.5%) | 1.000 | |
| Sex | Male | 52 (61.2%) | 51 (60.0%) | |
| Female | 33 (38.8%) | 34 (40.0%) | 1.000 | |
| BMI* (kg/m2) | <18.5 | 3 (3.5%) | 3 (3.5%) | |
| ≥18.5 | 82 (96.5%) | 82 (96.5%) | 1.000 | |
| TLC† (cells/mm3) | <900 | 6 (7.1%) | 11 (12.9%) | |
| ≥900 | 79 (92.9%) | 74 (87.1%) | 0.307 | |
| Albumin (g/dL) | <3.5 | 17 (20.5%) | 5 (5.9%) | |
| ≥3.5 | 66 (79.5%) | 80 (94.1%) | 0.006 | |
| NRS‡ 2002 high risk | No | 60 (70.6%) | 48 (56.5%) | |
| Yes | 25 (29.4%) | 37 (43.5%) | 0.079 | |
| Risk of undernutrition by MUST§ | Low | 85 (100%) | 62 (72.9%) | |
| Medium | 0 | 15 (17.6%) | ||
| High | 0 | 8 (9.4%) | <0.001 | |
| SNUBH-NST‖ high risk | No | 84 (98.8%) | 81 (95.3%) | |
| Yes | 1 (1.2%) | 4 (4.7%) | 0.368 |
| Morbidity (-) (N=155) | Morbidity (+)* (N=15) | P-value | ||
|---|---|---|---|---|
| Age (years) | ≤65 | 66 (90.4%) | 7 (9.6%) | 0.790 |
| >65 | 89 (91.8%) | 8 | ||
| Sex | Male | 92 (89.3%) | 11 (10.7%) | 0.409 |
| Female | 63 (94.0%) | 4 (6.0%) | ||
| BMI* (kg/m2) | <18.5 | 5 (83.3%) | 1 (16.7%) | 0.430 |
| ≥18.5 | 150 (91.5%) | 14 (8.5%) | ||
| TLC† (cells/mm3) | <900 | 15 (88.2%) | 2 (11.8%) | 0.649 |
| ≥900 | 140 (91.5%) | 13 (8.5%) | ||
| Albumin (g/dL) | <3.5 | 20 (90.9%) | 2 (9.1%) | 1.000 |
| ≥3.5 | 133 (91.1%) | 13 (89.9%) | ||
| NRS‡ 2002 high risk | No | 100 (92.6%) | 8 (7.4%) | 0.410 |
| Yes | 55 (88.7%) | 7 (11.3%) | ||
| Risk of undernutrition by MUST§ | Low | 133 (90.5%) | 14 (9.5%) | 0.622 |
| Medium | 14 (93.3%) | 1 (6.7%) | ||
| High | 8 (100%) | 0 | ||
| SNUBH-NST‖ high risk | No | 150 (90.9%) | 15 (9.1%) | 1.000 |
| Yes | 5 (100%) | 0 |
We divided our patients into two groups according to the presence or absence of morbidity for identifying the association between the preoperative nutritional status and postoperative complications. Patients with Dindo grade I or severe were included to morbidity positive group and patients without any complication to morbidity negative group.
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hospital Nutritional Screening Tool.
| Gastric cancer (N=85) | Colorectal cancer (N=85) | P-value | ||
|---|---|---|---|---|
| Postoperative hospital stay (days) | Mean±SD | 12.4±4.8 | 9.5±6.7 | 0.002 |
| BMI* (kg/m2) | <18.5 | 2 (2.4%) | 5 (5.9%) | 0.443 |
| ≥18.5 | 83 (97.6%) | 80 (94.1%) | ||
| TLC† (cells/mm3) | <900 | 15 (17.6%) | 15 (17.6%) | 1.000 |
| ≥900 | 70 (82.4%) | 70 (82.4%) | ||
| Albumin (g/dL) | <3.5 | 48 (57.1%) | 28 (32.9%) | 0.002 |
| ≥3.5 | 36 (42.9%) | 57 (67.1%) | ||
| NRS‡ 2002 high risk | No | 32 (37.6%) | 33 (38.8%) | 1.000 |
| Yes | 53 (62.4%) | 52 (61.2%) | ||
| Risk of undernutrition by MUST§ | Low | 32 (37.6%) | 51 (60.0%) | 0.007 |
| Medium | 24 (28.2%) | 20 (23.5%) | ||
| High | 29 (34.1%) | 14 (16.5%) | ||
| SNUBH-NST‖ high risk | No | 72 (84.7%) | 73 (85.9%) | 1.000 |
| Yes | 13 (15.3%) | 12 (14.1%) | ||
| Weight loss (5%) | No | 38 (44.7%) | 55 (64.7%) | 0.013 |
| Yes | 47 (55.3%) | 30 (35.3%) | ||
| Weight loss (10%) | No | 62 (72.9%) | 76 (89.4%) | 0.010 |
| Yes | 23 (27.1%) | 9 (10.6%) |
| Gastric cancer (%, N=85) | Colorectal cancer (%, N=85) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| TG¶ (N=18) | B-I** (N=40) | B-II†† (N=25) | P-value | RHC‡‡ (N=14) | LHC§§ (N=4) | AR‖‖ (N=30) | Proctectomy & TME¶¶ | P-value | |||
|
|
|||||||||||
| Without stoma (N=9) | With stoma (N=28) | ||||||||||
| Morbidity | No | 17 (94.4%) | 36 (90.0%) | 25 (100%) | 12 (85.7%) | 4 (100%) | 27 (90.0%) | 8 (88.9%) | 24 (85.7%) | ||
| Yes | 1 (5.6%) | 4 (10.0%) | 0 | 0.256 | 2 (14.3%) | 0 | 3 (10.0%) | 1 (11.1%) | 4 (14.3%) | 0.927 | |
| Postoperative hospital stay (days) | Mean±SD | 15.1±4.8 | 11.6±5.1 | 11.9±3.9 | 0.030 | 12.0±6.5 | 7.3±1.2 | 8.5±4.7 | 7.7±0.8 | 10.2±9.5 | 0.414 |
| BMI* (kg/m2) | <18.5 | 1 (5.6%) | 1 (2.5%) | 0 | 2 (14.3%) | 0 | 1 (3.3%) | 0 | 2 (7.1%) | ||
| ≥18.5 | 17 (94.4%) | 39 (97.5%) | 25 (100%) | 0.503 | 12 (85.7%) | 4 (100%) | 29 (96.7%) | 9 (100%) | 26 (92.9%) | 0.553 | |
| TLC† (cells/mm3) | <900 | 2 (11.1%) | 5 (12.5%) | 8 (32.0%) | 4 (28.6%) | 2 (50.0%) | 6 (20.0%) | 2 (22.2%) | 1 (3.6%) | ||
| ≥900 | 16 (88.9%) | 35 (87.5%) | 17 (68.0%) | 0.095 | 10 (71.4%) | 2 (50.0%) | 24 (80.0%) | 7 (77.8%) | 27 (96.4%) | 0.088 | |
| Albumin (g/dL) | <3.5 | 12 (66.7%) | 20 (50.0%) | 16 (64.0%) | 5 (35.7%) | 2 (50.0%) | 11 (36.7%) | 3 (33.3%) | 7 (25.0%) | ||
| ≥3.5 | 6 (33.3%) | 20 (50.0%) | 9 (36.0%) | 0.373 | 9 (64.3%) | 2 (50.0%) | 19 (63.3%) | 6 (66.7%) | 21 (75.0%) | 0.815 | |
| NRS‡ 2002 high risk | No | 3 (16.7%) | 19 (47.5%) | 10 (40.0%) | 8 (57.1%) | 2 (50.0%) | 14 (46.7%) | 2 (22.2%) | 7 (25.0%) | ||
| Yes | 15 (83.3%) | 21 (52.5%) | 15 (60.0%) | 0.082 | 6 (42.9%) | 2 (50.0%) | 16 (53.3%) | 7 (77.8%) | 21 (75.0%) | 0.18 | |
| Risk of undernutrition by MUST§ | Low | 3 (16.7%) | 20 (50.0%) | 9 (36.0%) | 10 (71.4%) | 4 | 18 (60.0%) | 6 (66.7%) | 13 (46.4%) | ||
| Medium | 3 (16.7%) | 12 (30.0%) | 7 (28.0%) | 1 (7.1%) | 0 | 10 (33.3%) | 1 (11.1%) | 8 (28.6%) | |||
| High | 12 (66.7%) | 8 (20.0%) | 9 (36.0%) | 0.016 | 3 (21.4%) | 0 | 2 (6.7%) | 2 (22.2%) | 7 (25.0%) | 0.202 | |
| SNUBH-NST‖ high risk | No | 16 (88.9%) | 35 (87.5%) | 20 (80.0%) | 9 (64.3%) | 3 (75.0%) | 26 (86.7%) | 7 (77.8%) | 28 (100%) | ||
| Yes | 2 (11.1%) | 5 (12.5%) | 5 (20.0%) | 0.635 | 5 (35.7%) | 1 (25.0%) | 4 (13.3%) | 2 (22.2%) | 0 | 0.028 | |
| Weight loss (5%) | No | 3 (16.7%) | 23 (57.5%) | 12 (48.0%) | 12 (85.7%) | 4 | 20 (66.7%) | 6 (66.7%) | 13 (46.4%) | ||
| Yes | 15 (83.3%) | 17 (42.5%) | 13 (52.0%) | 0.015 | 2 (14.3%) | 0 | 10 (33.3%) | 3 (33.3%) | 15 (53.6%) | 0.06 | |
| Weight loss (10%) | No | 7 (38.9%) | 35 (87.5%) | 18 (72.0%) | 13 (92.9%) | 4 | 30 (100%) | 7 (77.8%) | 22 (78.6%) | ||
| Yes | 11 (61.1%) | 5 (12.5%) | 7 (28.0%) | 0.001 | 1 (7.1%) | 0 | 0 | 2 (22.2%) | 6 (21.4%) | 0.062 | |
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hospital Nutritional Screening Tool;
¶Total gastrectomy;
**Billoth-I subtotal gastrectomy;
††Bilooth-II subtotal gastrectomy;
‡‡Right hemicolectomy;
§§Left hemicolectomy;
‖‖Anterior resection;
¶¶Total mesorectal excision.
DISCUSSION
- 1. Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CM, et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr 2013;32:65-72. ArticlePubMed
- 2. Bosaeus I. Nutritional support in multimodal therapy for cancer cachexia. Support Care Cancer 2008;16:447-51. ArticlePubMedPDF
- 3. Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, Builmaassen MT, Soeters PB. Perioperative nutritional support: a randomised clinical trial. Clin Nutr 1992;11:180-6. ArticlePubMed
- 4. Meguid MM, Curtas MS, Meguid V, Campos AC. Effects of pre-operative TPN on surgical risk--preliminary status report. Br J Clin Pract Suppl 1988;63:53-8. ArticlePubMed
- 5. Bozzetti F, Gavazzi C, Miceli R, Rossi N, Mariani L, Cozzaglio L, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr 2000;24:7-14. ArticlePubMed
- 6. Gustafsson UO, Ljungqvist O. Perioperative nutritional management in digestive tract surgery. Curr Opin Clin Nutr Metab Care 2011;14:504-9. ArticlePubMed
- 7. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. ArticlePubMed
- 8. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;2:40:205-13. Article
- 9. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. ArticlePubMed
- 10. Kim SY, Yeom HS, Park YM, Chung SH, Shin AR, Han HS, et al. Comparison of tools for nutritional risk screening at hospital admission. J Korean Soc Parenter Enter Nutr 2009;2:6-12. Article
- 11. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol 2016;42:1176-82. ArticlePubMed
- 12. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. ArticlePubMed
- 13. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat 2017;49:292-305. ArticlePubMedPMCPDF
- 14. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:783-800. ArticlePubMed
- 15. Makuuchi R, Sugisawa N, Kaji S, Hikage M, Tokunaga M, Tanizawa Y, et al. Enhanced recovery after surgery for gastric cancer and an assessment of preoperative carbohydrate loading. Eur J Surg Oncol 2017;43:210-7. ArticlePubMed
- 16. Shim H, Cheong JH, Lee KY, Lee H, Lee JG, Noh SH. Perioperative nutritional status changes in gastrointestinal cancer patients. Yonsei Med J 2013;54:1370-6. ArticlePubMedPMC
- 17. Kosuga T, Hiki N, Nunobe S, Noma H, Honda M, Tanimura S, et al. Feasibility and nutritional impact of laparoscopy-assisted subtotal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol 2014;21:2028-35. ArticlePubMedPDF
- 18. Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg 2011;35:357-64. ArticlePubMedPDF
- 19. Kye BH, Kim HJ, Kim JG, Cho HM. The nutritional impact of diverting stoma-related complications in elderly rectal cancer patients. Int J Colorectal Dis 2013;28:1393-400. ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

- Feasibility and Safety of Early Oral Feeding After Radical Gastrectomy in Patients With Gastric Carcinoma: A Systematic Review
Wahida Ali, Wahidullah Dost, Mohammad Nazir Zaman, Mohammad Qaher Rasully , Jamaluddin Niazi, Farzad Qasemi, Raisa Dost, Wahida Dost, Danyal Bakht, Syed Faqeer Hussain Bokhari
Cureus.2024;[Epub] CrossRef - Preoperative Body Mass Index, Waist Circumference, and Mortality After Major Cancer Surgery: A Nationwide Cohort Study in Korea
Tak Kyu Oh, In-Ae Song
Journal of Korean Medical Science.2023;[Epub] CrossRef - Nutritional Counseling Protocol for Colorectal Cancer Patients after Surgery Improves Outcome
Isabelle R. Novelli, Bruno A. D. Araújo, Laura F. Grandisoli, Elianete C. G. Furtado, Evelyn K. N. Aguchiku, Marina C. G. Bertocco, Tassiane P. Sudbrak, Isabel C. de Araújo, Ana C. F. Bosko, Nágila R. T. Damasceno
Nutrition and Cancer.2021; 73(11-12): 2278. CrossRef
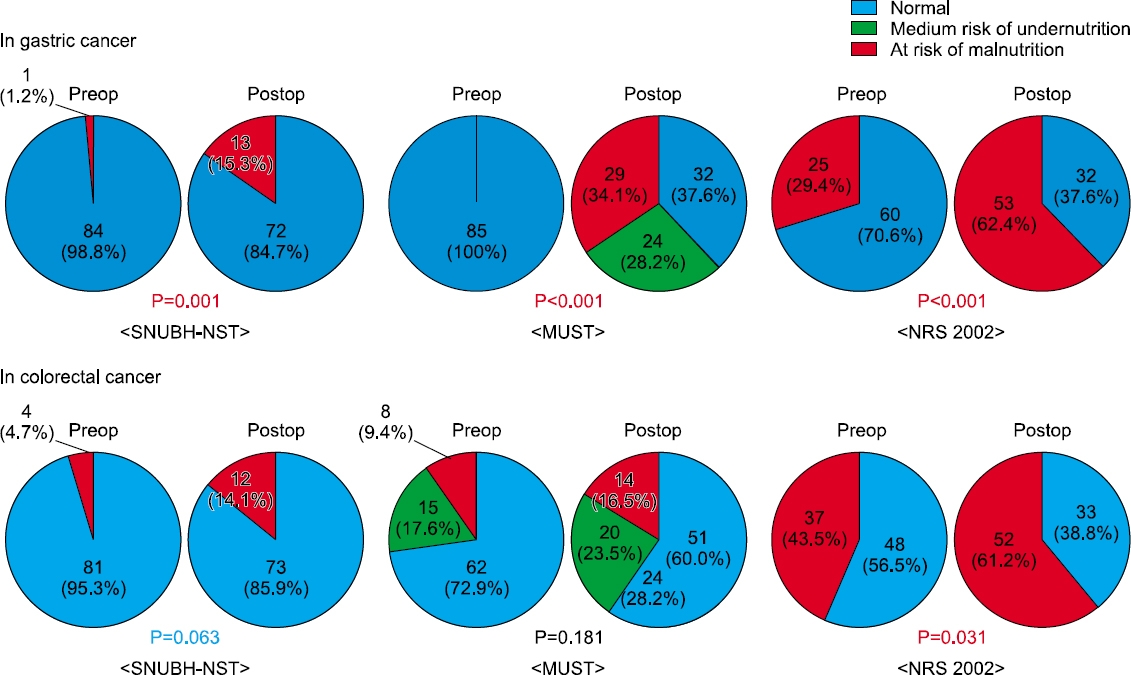
Fig. 1
Preoperative patient demographics
| Gastric cancer (N=85) | Colorectal cancer (N=85) | P-value | ||
|---|---|---|---|---|
| Age (years) | ≤65 | 49 (57.6%) | 48 (56.5%) | |
| >65 | 36 (42.4%) | 37 (43.5%) | 1.000 | |
| Sex | Male | 52 (61.2%) | 51 (60.0%) | |
| Female | 33 (38.8%) | 34 (40.0%) | 1.000 | |
| BMI |
<18.5 | 3 (3.5%) | 3 (3.5%) | |
| ≥18.5 | 82 (96.5%) | 82 (96.5%) | 1.000 | |
| TLC |
<900 | 6 (7.1%) | 11 (12.9%) | |
| ≥900 | 79 (92.9%) | 74 (87.1%) | 0.307 | |
| Albumin (g/dL) | <3.5 | 17 (20.5%) | 5 (5.9%) | |
| ≥3.5 | 66 (79.5%) | 80 (94.1%) | 0.006 | |
| NRS |
No | 60 (70.6%) | 48 (56.5%) | |
| Yes | 25 (29.4%) | 37 (43.5%) | 0.079 | |
| Risk of undernutrition by MUST |
Low | 85 (100%) | 62 (72.9%) | |
| Medium | 0 | 15 (17.6%) | ||
| High | 0 | 8 (9.4%) | <0.001 | |
| SNUBH-NST |
No | 84 (98.8%) | 81 (95.3%) | |
| Yes | 1 (1.2%) | 4 (4.7%) | 0.368 |
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hos pital Nutritional Screening Tool.
Pathologic staging and postoperative morbidity
| Gastric cancer (N=85) | Colorectal cancer (N=85) | P-value | ||
|---|---|---|---|---|
| T stage | 1 | 54 (63.5%) | 8 (9.4%) | |
| 2 | 8 (9.4%) | 13 (15.3%) | ||
| 3 | 10 (11.8%) | 48 (56.5%) | ||
| 4 | 13 (15.3%) | 16 (18.8%) | <0.001 | |
| Lymph node status | Negative | 58 (68.2%) | 44 (51.8%) | |
| Involved | 27 (31.8%) | 41 (48.2%) | 0.041 | |
| Overall TNM stage | I | 57 (67.1%) | 15 (17.6%) | |
| II | 12 (14.1%) | 29 (34.1%) | ||
| III | 16 (18.8%) | 41 (48.2%) | <0.001 | |
| Morbidity | No | 80 (94.1%) | 75 (88.2%) | |
| Yes | 5 (5.9%) | 10 (11.8%) | 0.279 | |
| Anastomosis problem | 2 cases | 2 | 2 | |
| Bleeding | 1 case | 1 | 3 | |
| Ileus | 2 cases | 2 | 5 | |
| Clavien-Dindo classification | Below 3 | 83 (97.6%) | 81 (95.3%) | 0.682 |
| 3 or Higher | 2 (2.4%) | 4 (4.7%) |
Preoperative nutritional state and postoperative morbidity
| Morbidity (-) (N=155) | Morbidity (+) |
P-value | ||
|---|---|---|---|---|
| Age (years) | ≤65 | 66 (90.4%) | 7 (9.6%) | 0.790 |
| >65 | 89 (91.8%) | 8 | ||
| Sex | Male | 92 (89.3%) | 11 (10.7%) | 0.409 |
| Female | 63 (94.0%) | 4 (6.0%) | ||
| BMI* (kg/m2) | <18.5 | 5 (83.3%) | 1 (16.7%) | 0.430 |
| ≥18.5 | 150 (91.5%) | 14 (8.5%) | ||
| TLC |
<900 | 15 (88.2%) | 2 (11.8%) | 0.649 |
| ≥900 | 140 (91.5%) | 13 (8.5%) | ||
| Albumin (g/dL) | <3.5 | 20 (90.9%) | 2 (9.1%) | 1.000 |
| ≥3.5 | 133 (91.1%) | 13 (89.9%) | ||
| NRS |
No | 100 (92.6%) | 8 (7.4%) | 0.410 |
| Yes | 55 (88.7%) | 7 (11.3%) | ||
| Risk of undernutrition by MUST |
Low | 133 (90.5%) | 14 (9.5%) | 0.622 |
| Medium | 14 (93.3%) | 1 (6.7%) | ||
| High | 8 (100%) | 0 | ||
| SNUBH-NST |
No | 150 (90.9%) | 15 (9.1%) | 1.000 |
| Yes | 5 (100%) | 0 |
We divided our patients into two groups according to the presence or absence of morbidity for identifying the association between the preoperative nutritional status and postoperative complications. Patients with Dindo grade I or severe were included to morbidity positive group and patients without any complication to morbidity negative group.
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hospital Nutritional Screening Tool.
Postoperative nutritional status at the time of discharge
| Gastric cancer (N=85) | Colorectal cancer (N=85) | P-value | ||
|---|---|---|---|---|
| Postoperative hospital stay (days) | Mean±SD | 12.4±4.8 | 9.5±6.7 | 0.002 |
| BMI |
<18.5 | 2 (2.4%) | 5 (5.9%) | 0.443 |
| ≥18.5 | 83 (97.6%) | 80 (94.1%) | ||
| TLC |
<900 | 15 (17.6%) | 15 (17.6%) | 1.000 |
| ≥900 | 70 (82.4%) | 70 (82.4%) | ||
| Albumin (g/dL) | <3.5 | 48 (57.1%) | 28 (32.9%) | 0.002 |
| ≥3.5 | 36 (42.9%) | 57 (67.1%) | ||
| NRS |
No | 32 (37.6%) | 33 (38.8%) | 1.000 |
| Yes | 53 (62.4%) | 52 (61.2%) | ||
| Risk of undernutrition by MUST |
Low | 32 (37.6%) | 51 (60.0%) | 0.007 |
| Medium | 24 (28.2%) | 20 (23.5%) | ||
| High | 29 (34.1%) | 14 (16.5%) | ||
| SNUBH-NST |
No | 72 (84.7%) | 73 (85.9%) | 1.000 |
| Yes | 13 (15.3%) | 12 (14.1%) | ||
| Weight loss (5%) | No | 38 (44.7%) | 55 (64.7%) | 0.013 |
| Yes | 47 (55.3%) | 30 (35.3%) | ||
| Weight loss (10%) | No | 62 (72.9%) | 76 (89.4%) | 0.010 |
| Yes | 23 (27.1%) | 9 (10.6%) |
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hospital Nutritional Screening Tool.
Postoperative nutritional status at the time of discharge and morbidity according to type of surgery
| Gastric cancer (%, N=85) | Colorectal cancer (%, N=85) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG |
B-I |
B-II |
P-value | RHC |
LHC |
AR |
Proctectomy & TME |
P-value | |||
| Without stoma (N=9) | With stoma (N=28) | ||||||||||
| Morbidity | No | 17 (94.4%) | 36 (90.0%) | 25 (100%) | 12 (85.7%) | 4 (100%) | 27 (90.0%) | 8 (88.9%) | 24 (85.7%) | ||
| Yes | 1 (5.6%) | 4 (10.0%) | 0 | 0.256 | 2 (14.3%) | 0 | 3 (10.0%) | 1 (11.1%) | 4 (14.3%) | 0.927 | |
| Postoperative hospital stay (days) | Mean±SD | 15.1±4.8 | 11.6±5.1 | 11.9±3.9 | 0.030 | 12.0±6.5 | 7.3±1.2 | 8.5±4.7 | 7.7±0.8 | 10.2±9.5 | 0.414 |
| BMI |
<18.5 | 1 (5.6%) | 1 (2.5%) | 0 | 2 (14.3%) | 0 | 1 (3.3%) | 0 | 2 (7.1%) | ||
| ≥18.5 | 17 (94.4%) | 39 (97.5%) | 25 (100%) | 0.503 | 12 (85.7%) | 4 (100%) | 29 (96.7%) | 9 (100%) | 26 (92.9%) | 0.553 | |
| TLC |
<900 | 2 (11.1%) | 5 (12.5%) | 8 (32.0%) | 4 (28.6%) | 2 (50.0%) | 6 (20.0%) | 2 (22.2%) | 1 (3.6%) | ||
| ≥900 | 16 (88.9%) | 35 (87.5%) | 17 (68.0%) | 0.095 | 10 (71.4%) | 2 (50.0%) | 24 (80.0%) | 7 (77.8%) | 27 (96.4%) | 0.088 | |
| Albumin (g/dL) | <3.5 | 12 (66.7%) | 20 (50.0%) | 16 (64.0%) | 5 (35.7%) | 2 (50.0%) | 11 (36.7%) | 3 (33.3%) | 7 (25.0%) | ||
| ≥3.5 | 6 (33.3%) | 20 (50.0%) | 9 (36.0%) | 0.373 | 9 (64.3%) | 2 (50.0%) | 19 (63.3%) | 6 (66.7%) | 21 (75.0%) | 0.815 | |
| NRS |
No | 3 (16.7%) | 19 (47.5%) | 10 (40.0%) | 8 (57.1%) | 2 (50.0%) | 14 (46.7%) | 2 (22.2%) | 7 (25.0%) | ||
| Yes | 15 (83.3%) | 21 (52.5%) | 15 (60.0%) | 0.082 | 6 (42.9%) | 2 (50.0%) | 16 (53.3%) | 7 (77.8%) | 21 (75.0%) | 0.18 | |
| Risk of undernutrition by MUST |
Low | 3 (16.7%) | 20 (50.0%) | 9 (36.0%) | 10 (71.4%) | 4 | 18 (60.0%) | 6 (66.7%) | 13 (46.4%) | ||
| Medium | 3 (16.7%) | 12 (30.0%) | 7 (28.0%) | 1 (7.1%) | 0 | 10 (33.3%) | 1 (11.1%) | 8 (28.6%) | |||
| High | 12 (66.7%) | 8 (20.0%) | 9 (36.0%) | 0.016 | 3 (21.4%) | 0 | 2 (6.7%) | 2 (22.2%) | 7 (25.0%) | 0.202 | |
| SNUBH-NST |
No | 16 (88.9%) | 35 (87.5%) | 20 (80.0%) | 9 (64.3%) | 3 (75.0%) | 26 (86.7%) | 7 (77.8%) | 28 (100%) | ||
| Yes | 2 (11.1%) | 5 (12.5%) | 5 (20.0%) | 0.635 | 5 (35.7%) | 1 (25.0%) | 4 (13.3%) | 2 (22.2%) | 0 | 0.028 | |
| Weight loss (5%) | No | 3 (16.7%) | 23 (57.5%) | 12 (48.0%) | 12 (85.7%) | 4 | 20 (66.7%) | 6 (66.7%) | 13 (46.4%) | ||
| Yes | 15 (83.3%) | 17 (42.5%) | 13 (52.0%) | 0.015 | 2 (14.3%) | 0 | 10 (33.3%) | 3 (33.3%) | 15 (53.6%) | 0.06 | |
| Weight loss (10%) | No | 7 (38.9%) | 35 (87.5%) | 18 (72.0%) | 13 (92.9%) | 4 | 30 (100%) | 7 (77.8%) | 22 (78.6%) | ||
| Yes | 11 (61.1%) | 5 (12.5%) | 7 (28.0%) | 0.001 | 1 (7.1%) | 0 | 0 | 2 (22.2%) | 6 (21.4%) | 0.062 | |
*Body mass index;
†Total lymphocyte count;
‡Nutritional Risk Screening;
§Malnutrition Universal Screening Tool;
‖Seoul National University Bundang Hospital Nutritional Screening Tool;
¶Total gastrectomy;
**Billoth-I subtotal gastrectomy;
††Bilooth-II subtotal gastrectomy;
‡‡Right hemicolectomy;
§§Left hemicolectomy;
‖‖Anterior resection;
¶¶Total mesorectal excision.
Body mass index; Total lymphocyte count; Nutritional Risk Screening; Malnutrition Universal Screening Tool; Seoul National University Bundang Hos pital Nutritional Screening Tool.
We divided our patients into two groups according to the presence or absence of morbidity for identifying the association between the preoperative nutritional status and postoperative complications. Patients with Dindo grade I or severe were included to morbidity positive group and patients without any complication to morbidity negative group. Body mass index; Total lymphocyte count; Nutritional Risk Screening; Malnutrition Universal Screening Tool; Seoul National University Bundang Hospital Nutritional Screening Tool.
Body mass index; Total lymphocyte count; Nutritional Risk Screening; Malnutrition Universal Screening Tool; Seoul National University Bundang Hospital Nutritional Screening Tool.
Body mass index; Total lymphocyte count; Nutritional Risk Screening; Malnutrition Universal Screening Tool; Seoul National University Bundang Hospital Nutritional Screening Tool; Total gastrectomy; Billoth-I subtotal gastrectomy; Bilooth-II subtotal gastrectomy; Right hemicolectomy; Left hemicolectomy; Anterior resection; Total mesorectal excision.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite

