Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 11(2); 2020 > Article
- Original Article Clinical Impact of Preoperative Sarcopenia to Postoperative Prognosis in Patients with Periampullary Malignancy: Retrospective Multicenter Study
-
Jee Hyun Park, M.D.1
 , Youngju Ryu, M.D.1
, Youngju Ryu, M.D.1 , So Hee Song, M.D.2
, So Hee Song, M.D.2 , Naru Kim, M.D.1
, Naru Kim, M.D.1 , Sang Hyun Shin, M.D., Ph.D.1
, Sang Hyun Shin, M.D., Ph.D.1 , Jin Seok Heo, M.D., Ph.D.1
, Jin Seok Heo, M.D., Ph.D.1 , Dong Wook Choi, M.D., Ph.D.1
, Dong Wook Choi, M.D., Ph.D.1 , Woo Kyoung Jeong, M.D., Ph.D.2
, Woo Kyoung Jeong, M.D., Ph.D.2 , Woo Hyun Jung, M.D.3
, Woo Hyun Jung, M.D.3 , Yong Chan Shin, M.D.4
, Yong Chan Shin, M.D.4 , Chang-Sup Lim, M.D., Ph.D.5
, Chang-Sup Lim, M.D., Ph.D.5 , In Woong Han, M.D., Ph.D.1
, In Woong Han, M.D., Ph.D.1
-
Surgical Metabolism and Nutrition 2020;11(2):40-45.
DOI: https://doi.org/10.18858/smn.2020.11.2.40
Published online: December 30, 2020
1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Radiology Centre for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Surgery, Ajou University Medical Center, Ajou University College of Medicine, Suwon, Korea
4Department of Surgery, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
5Department of Surgery, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- Corresponding author: In Woong Han E-mail cardioman76@gmail.com ORCID https://orcid.org/0000-0001-7093-2469
This study was supported by grant from the KSSMN grant no. 2017-04 and presented at 29th Congress of the KSSMN & 2019 International Symposium, Seoul, Korea.
Copyright © 2020 The Korean Society of Surgical Metabolism and Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 659 Views
- 0 Download
Abstract
-
Purpose This study compared the preoperative nutritional status between sarcopenic and non-sarcopenic patients and examined the effects of sarcopenia on the prognosis after a pancreatoduodenectomy (PD).
-
Materials and Methods From 2015 to 2016, 480 patients who underwent PD with periampullary cancer at Samsung Medical Center, Seoul National University Boramae Medical Center, Ilsan Paik Hospital, and Ajou University Hospital were analyzed retrospectively. Sarcopenia was measured from the cross-sectional visceral fat and muscle area on CT imaging using an automatic calculation program. The dysnutritional grade was assessed according to Controlling Nutritional Status (CONUT) score system.
-
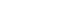
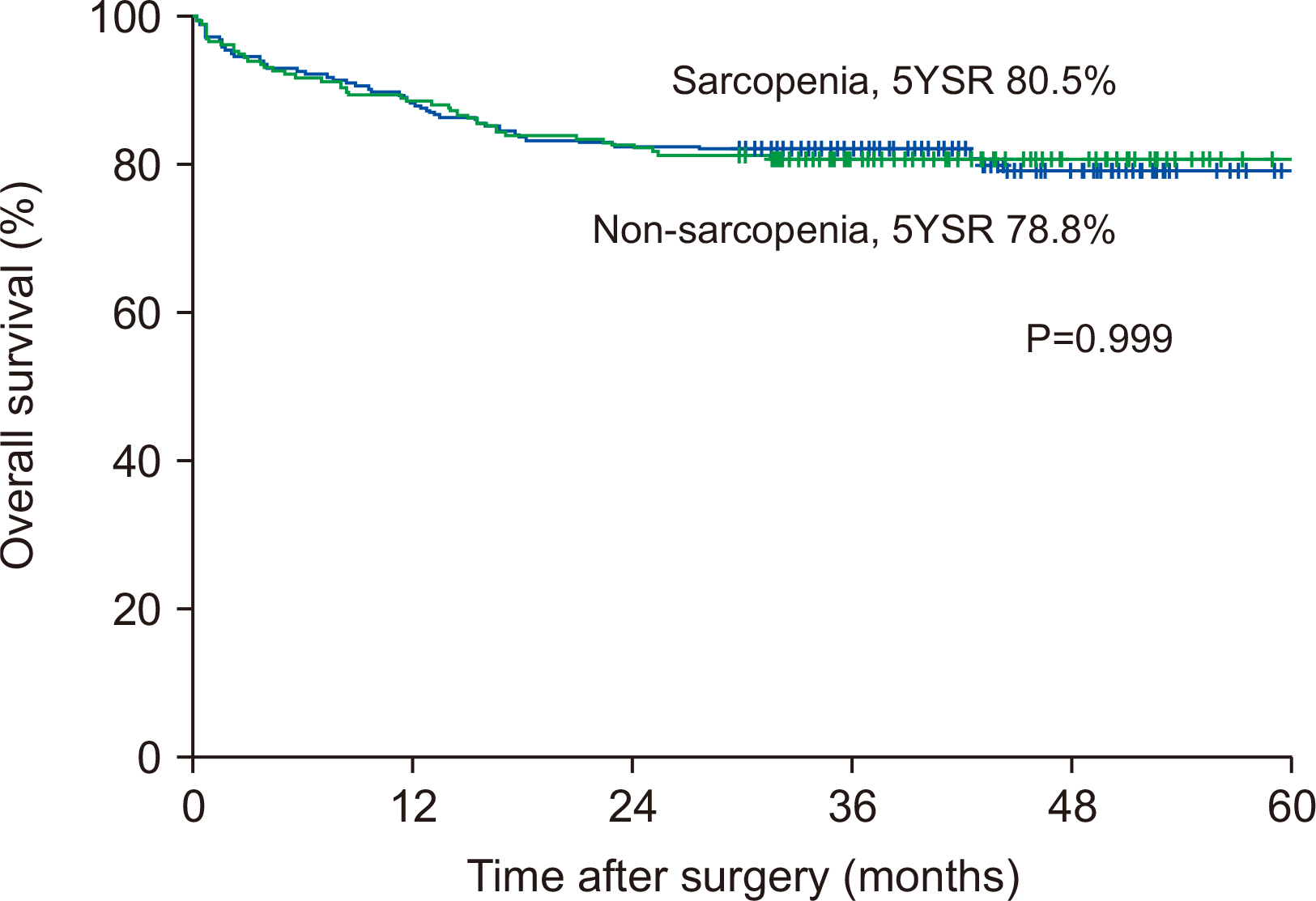
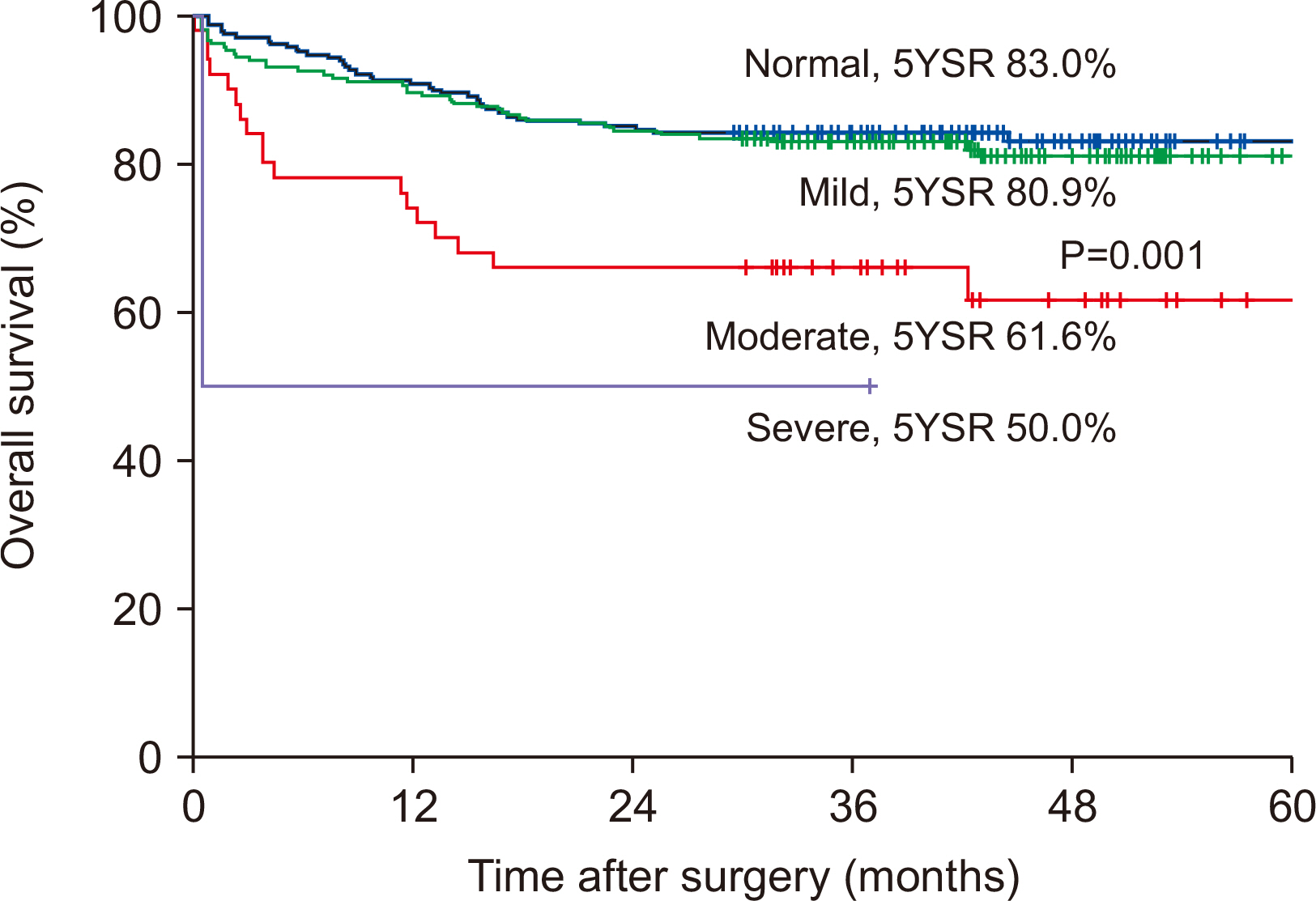
Results Preoperative serum albumin (3.9 g/dl) and cholesterol levels (161.7 mg/dl) of sarcopenic patients were significantly lower than those of the non-sarcopenia patients (4.0 g/dl, P=0.024; 176.1 mg/dl, P=0.005). The proportion of moderate-to-severe dysnutritional grade in sarcopenic patients was significantly higher than in the non-sarcopenic patients (20.0 vs. 8.1%, P=0.004). A comparison of the changes in albumin between before and after PD showed a decrease in sarcopenic patients (0.06 vs. 0.05, P=0.024). Sarcopenia itself was not a factor affecting the overall survival (OS) negatively, but moderate-to-severe dysnutritional grade was an independent risk factor for OS (HR 2.418, CI 1.424~4.107, P=0.001).
-
Conclusion Patients with sarcopenia showed poorer preoperative nutritional status than those without sarcopenia, and the sarcopenia affected the postoperative nutritional status negatively. No direct correlation was observed between sarcopenia and OS, but the dysnutritional grade was an independent risk factor that affects OS. As a result, patients with sarcopenia could be affected indirectly for survival because of their poor nutritional status.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
ACKNOWLEDGMENTS
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127(5 Suppl):990S-1S. ArticlePubMed
- 2. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. 2010;European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39:412-23. ArticlePubMedPMC
- 3. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249-56. ArticlePubMedPMC
- 4. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. ArticlePubMed
- 5. Turaga K, Kaushik M, Forse RA, Sasson AR. In hospital outcomes after pancreatectomies: an analysis of a national database from 1996 to 2004. J Surg Oncol 2008;98:156-60. ArticlePubMed
- 6. Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R. Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg 2018;267:411-7. ArticlePubMed
- 7. La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol 2013;107:702-8. ArticlePubMedPDF
- 8. Bozzetti F, Mariani L. Perioperative nutritional support of patients undergoing pancreatic surgery in the age of ERAS. Nutrition 2014;30:1267-71. ArticlePubMed
- 9. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One 201712::e0186990. Article
- 10. Tian S, Xu Y. Association of sarcopenicobesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int 2016;16:155-66. ArticlePubMed
- 11. Shen Y, Hao Q, Zhou J, Dong B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr 2017;17:188.ArticlePubMedPMCPDF
- 12. Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg 2017;41:2266-79. ArticlePubMedPMCPDF
- 13. Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. ArticlePubMedPMCPDF
- 14. Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2015;111:771-5. ArticlePubMedPDF
- 15. Moon JH, Kim KM, Kim JH, Moon JH, Choi SH, Lim S, et al. Predictive Values of the new sarcopenia index by the Foundation for the National Institutes of Health Sarcopenia Project for mortality among older Korean adults. PLoS One 2016;11:e0166344. ArticlePubMedPMC
- 16. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. 2008;A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997-1006. ArticlePubMed
- 17. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. Article
- 18. Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res 2017;29:43-8. ArticlePubMedPDF
- 19. Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 202011:366-80. ArticlePDF
- 20. Park JW, Jang JY, Kim EJ, Kang MJ, Kwon W, Chang YR, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg 2013;100:1064-70. ArticlePubMedPDF
- 21. Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. Prospective assessment of the influence of pancreatic cancer resection on exocrine pancreatic function. Br J Surg 2014;101:109-13. ArticlePubMedPDF
- 22. Nikfarjam M, Wilson JS, Smith RC. Australasian Pancreatic Club Pancreatic Enzyme Replacement Therapy Guidelines Working Group. Diagnosis and management of pancreatic exocrine insufficiency. Med J Aust 2017;207:161-5. ArticlePubMedPDF
- 23. Roberts KJ, Schrem H, Hodson J, Angelico R, Dasari BVM, Coldham CA, et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB (Oxford) 2017;19:859-67. ArticlePubMed
- 24. Onesti JK, Wright GP, Kenning SE, Tierney MT, Davis AT, Doherty MG, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology 2016;16:284-9. ArticlePubMed
- 25. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. ArticlePubMed
- 26. Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 2017;103:16-22. ArticlePubMed
- 27. Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 2015;19:1593-602. ArticlePubMedPMCPDF
- 28. Pecorelli N, Capretti G, Sandini M, Damascelli A, Cristel G, De Cobelli F, et al. Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomyfor cancer: results from a multicenter study. Ann Surg Oncol 2018;25:308-17. ArticlePubMedPDF
- 29. Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc 2008;56:1710-5. ArticlePubMed
- 30. Kyle UG, Genton L, Hans D, Karsegard VL, Michel JP, Slosman DO, et al. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc 2001;49:1633-40. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

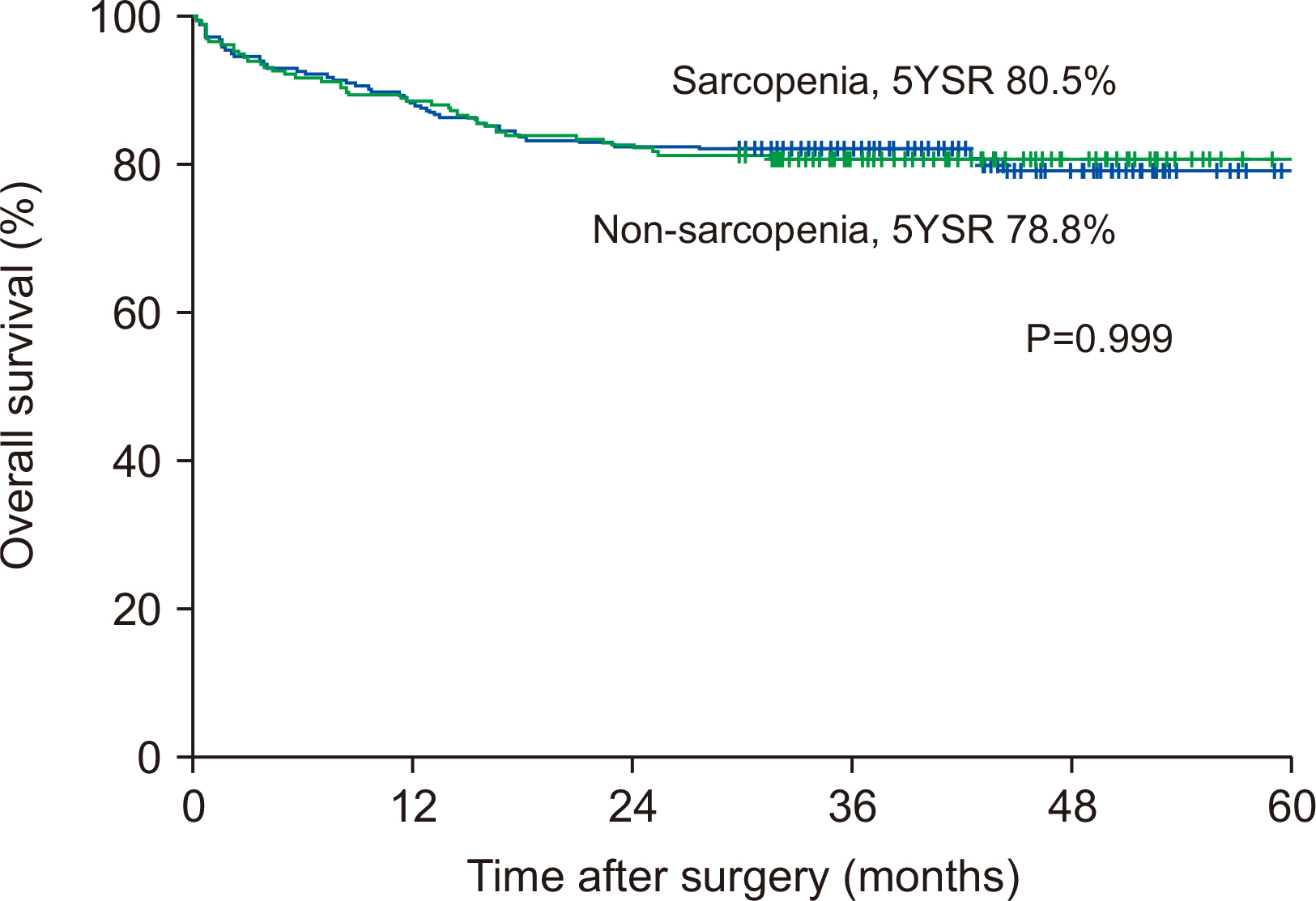
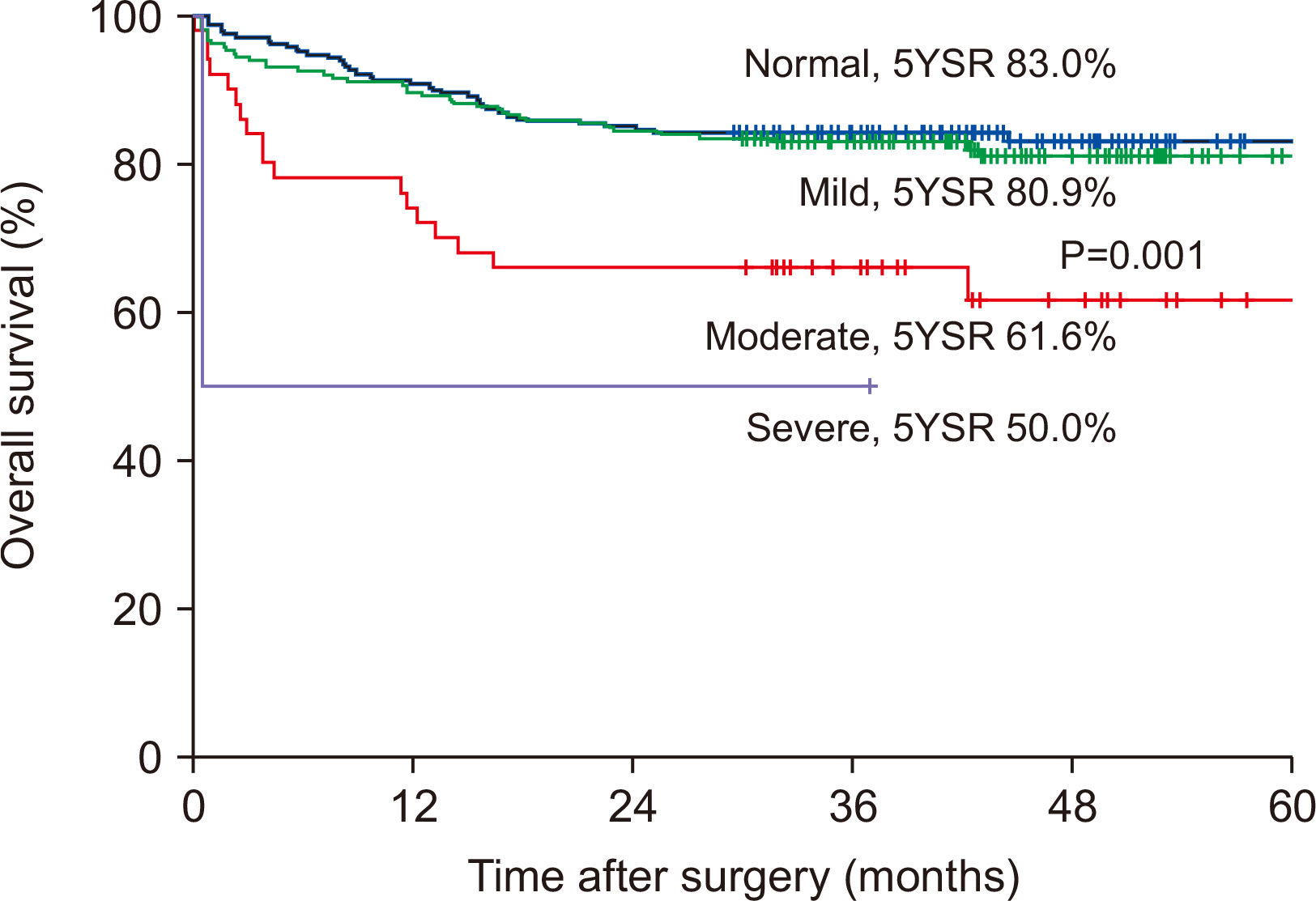
Fig. 1
Fig. 2
Characteristics of enrolled patients
| Total (n=480) | Non-sarcopenia (n=259) | Sarcopenia (n=221) | P | |
|---|---|---|---|---|
| Age (years) | 64.5 (14~92) | 64.0 (31~92) | 65.2 (14~89) | 0.189 |
| Sex | <0.001 | |||
| Male, n (%) | 272 (56.7) | 103 (37.9) | 169 (62.1) | |
| Female, n (%) | 208 (43.3) | 156 (75.0) | 52 (25.0) | |
| Body weight (kg) | 61.3 (31.4~95.5) | 62.6 (38.4~95.5) | 59.8 (31.4~88.0) | 0.007 |
| BMI (kg/m2) | 23.3 (14.2~38.5) | 24.6 (17.3~38.5) | 21.9 (14.2~30.0) | <0.001 |
| Serum albumin, preoperative (g/dl) | 3.92 (2.1~5.4) | 4.0 (2.4~5.4) | 3.9 (2.1~5.0) | 0.024 |
| Serum cholesterol, preoperative (mg/dl) | 170.3 (1~508) | 176.1 (39~508) | 161.7 (1~295) | 0.005 |
| Dysnutritional grade | 0.004 | |||
| Normal | 222 (46.3) | 133 (51.4) | 89 (40.3) | |
| Mild | 206 (42.9) | 105 (40.5) | 101 (45.7) | |
| Moderate | 50 (10.4) | 21 (8.1) | 29 (13.1) | |
| Severe | 2 (0.4) | 0 (0) | 2 (0.9) | |
| Diseases | 0.372 | |||
| Pancreas head cancer | 203 (42.3) | 112 (43.2) | 91 (41.2) | |
| Distal CBD cancer | 130 (27.1) | 66 (25.5) | 64 (29.0) | |
| Mid CBD cancer | 22 (4.6) | 15 (5.8) | 7 (3.2) | |
| AoV cancer | 99 (20.6) | 56 (21.6) | 43 (19.5) | |
| Duodenal cancer | 20 (4.2) | 8 (3.1) | 12 (5.4) | |
| Surgery | ||||
| PPPD | 351 (73.1) | 189 (73.0) | 162 (73.3) | |
| PRPD | 80 (16.7) | 49 (18.9) | 31 (14.0) | |
| Whipple’s operation | 47 (9.8) | 21 (8.1) | 26 (11.8) |
BMI = body mass index; CBD = common bile duct; AoV = ampulla of Vater; PPPD = pylorus-preserving pancreatoduodenectomy; PRPD = pylorus-resecting pancreatoduodenectomy.
Perioperative change of nutritional parameters in patients with or without sarcopenia
| Variables difference | Total (n=480) | Non-sarcopenia (n=259) | Sarcopenia (n=221) | P |
|---|---|---|---|---|
| Δ Body weight | 3.67 (–47.10~39.80) | 3.29 (–47.10~26.75) | 4.01 (–15.54~39.8) | 0.493 |
| Δ BMI | 1.34 (–21.80~11.63) | 1.38 (–21.80~10.62) | 1.26 (–8.34~11.63) | 0.075 |
| Δ Albumin | 0.05 (–1.9~3.6) | 0.05 (–1.6~3.6) | 0.06 (–1.9~1.8) | 0.024 |
| Δ Cholesterol | 30.71 (–208~275) | 34.04 (–208~275) | 25.84 (–146~156) | 0.948 |
Δ Value at preoperative time minus value at postoperative 6 months.
BMI = Body mass index.
Uni- and multivariate risk factors analysis for overall survival
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.014 | 0.994~1.035 | 0.167 | |||
| Male | 1.250 | 0.824~1.894 | 0.294 | |||
| BMI | 0.979 | 0.917~1.046 | 0.529 | |||
| Preoperative low albumin level (<3.5 g/dl) | 1.647 | 1.105~1.463 | 0.014 | 1.048 | 0.570~1.598 | 0.859 |
| Preoperative low cholesterol level (<170 mg/dl) | 1.004 | 1.001~1.009 | 0.046 | 1.002 | 0.993~1.002 | 0.295 |
| Sarcopenia | 1.000 | 0.666~1.501 | 0.999 | |||
| Dysnutritional grade (≥moderate, severe) | 2.546 | 1.538~4.214 | <0.001 | 2.418 | 1.424~4.107 | 0.001 |
BMI = Body mass index.
BMI = body mass index; CBD = common bile duct; AoV = ampulla of Vater; PPPD = pylorus-preserving pancreatoduodenectomy; PRPD = pylorus-resecting pancreatoduodenectomy.
Δ Value at preoperative time minus value at postoperative 6 months. BMI = Body mass index.
BMI = Body mass index.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite