Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 14(1); 2022 > Article
- Original Article Development of the Korean Version of the Gastrointestinal Quality of Life Index Questionnaire
-
In Jun Yang, M.D.1
 , Heung-Kwon Oh, M.D., Ph.D.1
, Heung-Kwon Oh, M.D., Ph.D.1 , Jeehye Lee, M.D.1
, Jeehye Lee, M.D.1 , Jung Wook Suh, M.D.1
, Jung Wook Suh, M.D.1 , Hong-min Ahn, M.D.1
, Hong-min Ahn, M.D.1 , Hyeonjeong Park, B.S.1
, Hyeonjeong Park, B.S.1 , Hyun Hee Sim, B.S.1
, Hyun Hee Sim, B.S.1 , Yong Beom Cho, M.D., Ph.D.2
, Yong Beom Cho, M.D., Ph.D.2 , In Kyu Lee, M.D., Ph.D.3
, In Kyu Lee, M.D., Ph.D.3 , Seungbum Ryoo, M.D., Ph.D.4
, Seungbum Ryoo, M.D., Ph.D.4 , Dong-Won Lee, M.D.5
, Dong-Won Lee, M.D.5 , Duck-Woo Kim, M.D., Ph.D.1
, Duck-Woo Kim, M.D., Ph.D.1 , Sung-Bum Kang, M.D., Ph.D.1
, Sung-Bum Kang, M.D., Ph.D.1
-
DOI: https://doi.org/10.15747/ACNM.2022.14.1.32
Published online: June 1, 2022
1Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Surgery, Samsung Medical Center, Seoul, Korea
3Department of Surgery, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea
4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
5Center for Colorectal Cancer, National Cancer Center, Goyang, Korea
- Corresponding author: Heung-Kwon Oh E-mail crsohk@gmail.com ORCID https://orcid.org/0000-0002-8066-2367
© The Korean Society of Surgical Metabolism and Nutrition and The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,403 Views
- 19 Download
- 2 Crossref
Abstract
-
Purpose To establish a standardized quality of life measurement that allows global cross-study comparisons, we translated the Gastrointestinal Quality of Life Index (GIQLI) into Korean and linguistically validated the Korean version of the GIQLI (K-GIQLI) in patients who underwent colorectal surgery.
-
Materials and Methods A cross-cultural adaptation of the original GIQLI was created based on the established guidelines. Based on participation in a cognitive interview, 20 patients with colorectal cancer were enrolled in the study. To ensure that the Korean version of the questionnaire was understood as intended, the time needed to complete the questionnaire was measured, and three additional items related to comprehension were added.
-
Results From May to July 2021, two translators, whose native language was Korean translated the GIQLI items into Korean, and a native English editor who had no knowledge of the original questionnaire translated the items back into English. In the cognitive interview, the median age of the patients was 61.8 (range: 44~82) years, and the median time required to complete the questionnaire was 6.5 (range: 5~10) min. For the language and cultural adaptation process, the participants’ comprehension of the questionnaire was measured on a scale of 1~5, with a mean score of 4 (range: 3~4).
-
Conclusion The K-GIQLI was developed and did not exhibit a significant difference from the original English version in terms of social, linguistic, and cultural differences between the Western world and Republic of Korea.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
AUTHOR CONTRIBUTIONS
Conceptualization: HKO. Data curation: HP, HHS. Formal analysis: IJY. Funding acquisition: HKO. Investigation: JWS, JL, HA. Methodology: IJY, HA, HP, HHS. Project administration: HKO. Resources: JWS, JL, HA. Supervision: YBC, IKL, SR. Validation: DWK, SBK. Visualization: DWL. Writing – original draft: IJY. Writing – review and editing: IJY, HKO.
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
FUNDING
The authors received financial support from the Korean Society of Surgical Metabolism and Nutrition.
| Subscales | Question number | Korean version |
|---|---|---|
| Symptoms (19 items) | 1 |
지난 2주간, 복통이 얼마나 자주 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| 2 |
지난 2주간, 윗배가 가득 찬 느낌으로 얼마나 자주 불편했습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 3 |
지난 2주간, 배에 가스가 가득 찬 느낌이나 팽만감으로 얼마나 자주 불편함을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 4 |
지난 2주간, 방귀로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 5 |
지난 2주간, 트림으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 6 |
지난 2주간, 위나 장에서 나는 이상한 소리를 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 7 |
지난 2주간, 빈번한 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 8a |
지난 2주간, 음식 먹는 것을 얼마나 자주 즐겼습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 9 |
당신의 질병 때문에 평소 좋아하는 음식을 먹지 못하고 참는 경우가 얼마나 자주 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 27 |
지난 2주간, 음식물의 역류로 불편함을 느낀 적이 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 28 |
지난 2주간, 느린 식사 속도로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 29 |
지난 2주간, 음식물을 삼킬 때의 어려움으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 30 |
지난 2주간, 급작스러운 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 31 |
지난 2주간, 설사로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 32 |
지난 2주간, 변비로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 33 |
지난 2주간, 구역질로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 34 |
지난 2주간, 변에 피가 섞여 나와서 얼마나 자주 놀랐습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 35 |
지난 2주간, 속쓰림으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 36 |
지난 2주간, 의도하지 않은 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| Subscales | Question number | Korean version |
|---|---|---|
| Emotions (5 items) | 10 |
지난 2주간, 일상적인 스트레스에 어떻게 대처했습니까? ① 아주 나쁘게 ② 나쁘게 ③ 적당히 ④ 좋게 ⑤ 아주 좋게 |
| 11 |
지난 2주간, 당신이 아프다는 사실 때문에 얼마나 자주 슬펐습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 12 |
지난 2주간, 당신의 질병으로 인해 얼마나 자주 긴장하거나 예민해졌습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 13a |
지난 2주간, 당신의 삶 전반에 대해 얼마나 자주 만족했습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 14 |
지난 2주간, 당신의 질병으로 좌절감을 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| Subscales | Question number | Korean version |
|---|---|---|
| Social function (4 items) | 22a |
지난 2주간, 직장, 학업, 집안일 등 정상적인 일상 활동을 유지할 수 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| 23a |
지난 2주간, 운동이나 취미생활 등 정상적인 여가 활동을 유지할 수 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 25 |
당신의 질병이 가까운 사람들과의 관계를 얼마나 많이 바꿨습니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| 26 |
당신의 질병이 본인의 성생활에 얼마나 나쁜 영향을 미쳤습니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| Medical treatment (1 item) | 24 |
지난 2주간, 치료로 인해 구속감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
Values are presented as median (interquartile range) or number (%).
Question 1 = “How long did you take to complete the questionnaire?”. Question 2 = “Are the meanings of the questions clear and unambiguous?”. Question 3= “ Are the questions in the questionnaire adequately described in terms of expressions used in daily life?”.
GIQLI = Gastrointestinal Quality of Life Index.
- 1. Malcolm FL, Adiamah A, Banerjea A, Whitehead D, Gupta A, West J, et al. ; Nottingham Colorectal Service. Long-term health-related quality of life following colorectal cancer surgery: patient-reported outcomes in a remote follow-up population. Colorectal Dis 2021;23:213-25. ArticlePubMed
- 2. Kopp M, Bonatti H, Haller C, Rumpold G, Söllner W, Holzner B, et al. Life satisfaction and active coping style are important predictors of recovery from surgery. J Psychosom Res 2003;55:371-7. ArticlePubMed
- 3. Maartense S, Dunker MS, Slors JF, Cuesta MA, Pierik EG, Gouma DJ, et al. Laparoscopic-assisted versus open ileocolic resection for Crohn's disease: a randomized trial. Ann Surg 2006;243:143-9. discussion 150-3. ArticlePubMedPMC
- 4. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. ArticlePubMed
- 5. van de Wall BJM, Stam MAW, Draaisma WA, Stellato R, Bemelman WA, Boermeester MA, et al. ; DIRECT trial collaborators. Surgery versus conservative management for recurrent and ongoing left-sided diverticulitis (DIRECT trial): an open-label, multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2017;2:13-22. ArticlePubMed
- 6. Mari GM, Crippa J, Cocozza E, Berselli M, Livraghi L, Carzaniga P, et al. Low ligation of inferior mesenteric artery in laparoscopic anterior resection for rectal cancer reduces genitourinary dysfunction: results from a randomized controlled trial (HIGHLOW trial). Ann Surg 2019;269:1018-24. ArticlePubMed
- 7. Ihn MH, Lee SM, Son IT, Park JT, Oh HK, Kim DW, et al. Cultural adaptation and validation of the Korean version of the EORTC QLQ-CR29 in patients with colorectal cancer. Support Care Cancer 2015;23:3493-501. ArticlePubMedPDF
- 8. Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg 1995;82:216-22. ArticlePubMedPDF
- 9. Jung YS. Trends in healthcare costs for inflammatory bowel disease in South Korea. Gut Liver 2020;14:3-4. ArticlePubMedPMC
- 10. Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 2017;36:321-47. ArticlePubMed
- 11. Ryan JJ, Hanes DA, Bradley RD, Contractor N. Effect of a nutrition support formula in adults with inflammatory bowel disease: a pilot study. Glob Adv Health Med 2019;8:2164956119867251. ArticlePubMedPMCPDF
- 12. Theodoropoulos GE, Memos NA, Peitsidou K, Karantanos T, Spyropoulos BG, Zografos G. 2016;Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol 29:56-62.ArticlePubMedPMC
- 13. Kim LS, Hilli L, Orlowski J, Kupperman JL, Baral M. F Waters R. Efficacy of probiotics and nutrients in functional gastrointestinal disorders: a preliminary clinical trial. Dig Dis Sci 2006;51:2134-44. ArticlePubMedPDF
- 14. Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut 2000;47:444-54. ArticlePubMedPMC
- 15. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Med Care 1981;19:787-805. ArticlePubMed
- 16. Shi HY, Lee HH, Chiu CC, Chiu HC, Uen YH, Lee KT. Responsiveness and minimal clinically important differences after cholecystectomy: GIQLI versus SF-36. J Gastrointest Surg 2008;12:1275-82. ArticlePubMedPDF
- 17. Yeung SM, Shiu AT, Martin CR, Chu KM. Translation and validation of the Chinese version of the Gastrointestinal Quality of Life Index in patients with gastric tumor. J Psychosom Res 2006;61:469-77. ArticlePubMed
- 18. Lien HH, Huang CC, Wang PC, Chen YH, Huang CS, Lin TL, et al. Validation assessment of the Chinese (Taiwan) version of the Gastrointestinal Quality of Life Index for patients with symptomatic gallstone disease. J Laparoendosc Adv Surg Tech A 2007;17:429-34. ArticlePubMed
- 19. Sandblom G, Videhult P, Karlson BM, Wollert S, Ljungdahl M, Darkahi B, et al. Validation of Gastrointestinal Quality of Life Index in Swedish for assessing the impact of gallstones on health-related quality of life. Value Health 2009;12:181-4. ArticlePubMed
- 20. Nieveen Van Dijkum EJ, Terwee CB, Oosterveld P, Van Der Meulen JH, Gouma DJ, De Haes JC. Validation of the Gastrointestinal Quality of Life Index for patients with potentially operable periampullary carcinoma. Br J Surg 2000;87:110-5. ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

- Comparison of laparoscopic and robotic surgery of choledochal cyst in pediatrics: single center experience
Jiyong Jang, Dayoung Ko, Joong Kee Youn, Hee-Beom Yang, Hyun-Young Kim
Surgical Endoscopy.2026; 40(1): 462. CrossRef - Longitudinal quality of life assessment after laparoscopic colorectal cancer surgery using the Gastrointestinal Quality of Life Index questionnaire: A multicentre prospective study
Tae‐Gyun Lee, Seung‐Bum Ryoo, Heung‐Kwon Oh, Yong Beom Cho, Chang Hyun Kim, Ju Hyun Lee, Hong‐Min Ahn, Hye‐Rim Shin, Mi Jeong Choi, Min Hyeong Jo, Duck‐Woo Kim, Sung‐Bum Kang
Colorectal Disease.2025;[Epub] CrossRef
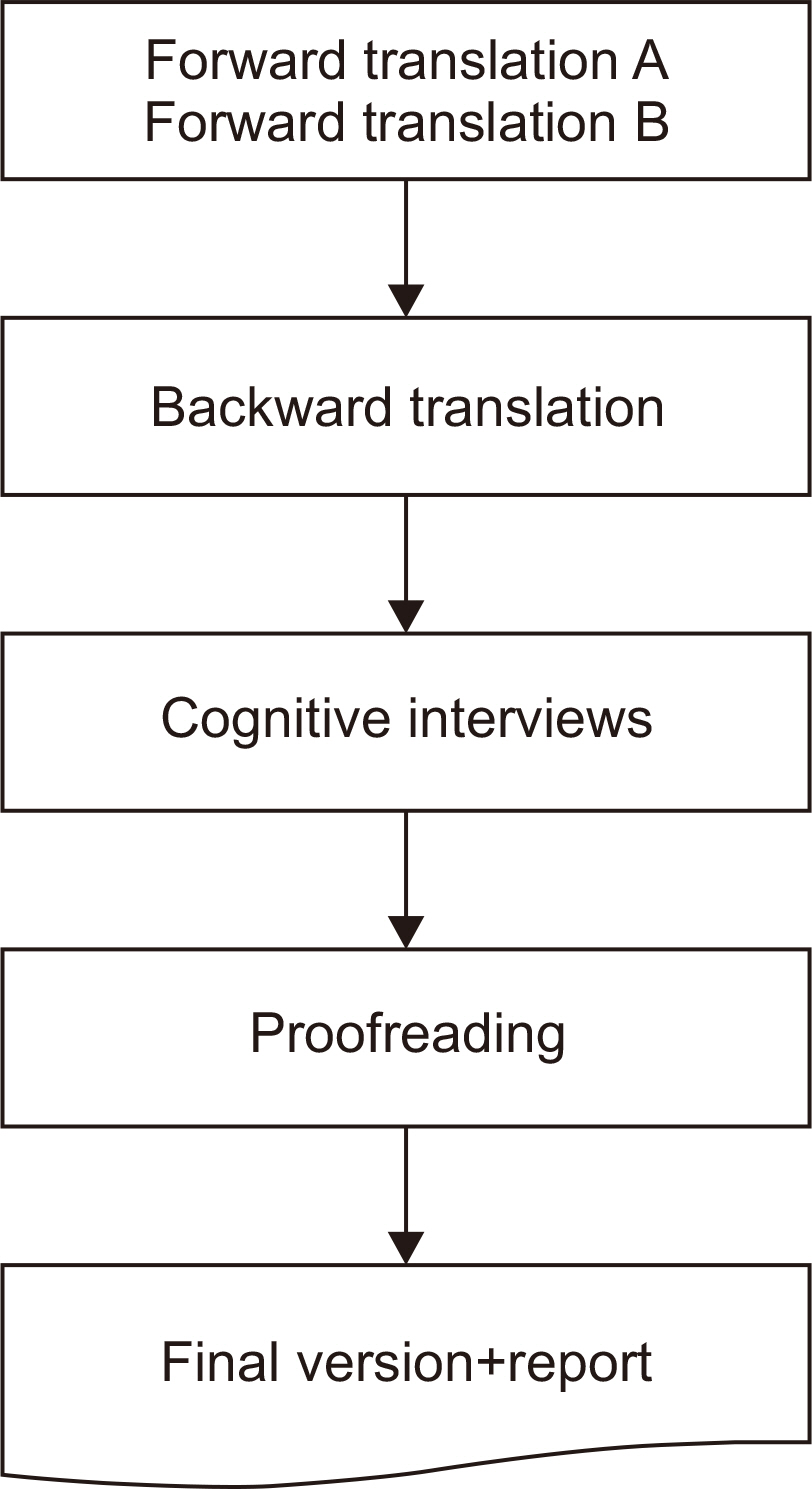
Fig. 1
Symptom subscale of the Korean version of the Gastrointestinal Quality of Life Index
| Subscales | Question number | Korean version |
|---|---|---|
| Symptoms (19 items) | 1 | 지난 2주간, 복통이 얼마나 자주 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| 2 | 지난 2주간, 윗배가 가득 찬 느낌으로 얼마나 자주 불편했습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 3 | 지난 2주간, 배에 가스가 가득 찬 느낌이나 팽만감으로 얼마나 자주 불편함을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 4 | 지난 2주간, 방귀로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 5 | 지난 2주간, 트림으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 6 | 지난 2주간, 위나 장에서 나는 이상한 소리를 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 7 | 지난 2주간, 빈번한 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 8 |
지난 2주간, 음식 먹는 것을 얼마나 자주 즐겼습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 9 | 당신의 질병 때문에 평소 좋아하는 음식을 먹지 못하고 참는 경우가 얼마나 자주 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 27 | 지난 2주간, 음식물의 역류로 불편함을 느낀 적이 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 28 | 지난 2주간, 느린 식사 속도로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 29 | 지난 2주간, 음식물을 삼킬 때의 어려움으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 30 | 지난 2주간, 급작스러운 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 31 | 지난 2주간, 설사로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 32 | 지난 2주간, 변비로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 33 | 지난 2주간, 구역질로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 34 | 지난 2주간, 변에 피가 섞여 나와서 얼마나 자주 놀랐습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 35 | 지난 2주간, 속쓰림으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 36 | 지난 2주간, 의도하지 않은 배변(대변보기)으로 얼마나 자주 불편감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Emotion subscale of the Korean version of the Gastrointestinal Quality of Life Index
| Subscales | Question number | Korean version |
|---|---|---|
| Emotions (5 items) | 10 | 지난 2주간, 일상적인 스트레스에 어떻게 대처했습니까? ① 아주 나쁘게 ② 나쁘게 ③ 적당히 ④ 좋게 ⑤ 아주 좋게 |
| 11 | 지난 2주간, 당신이 아프다는 사실 때문에 얼마나 자주 슬펐습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 12 | 지난 2주간, 당신의 질병으로 인해 얼마나 자주 긴장하거나 예민해졌습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 13 |
지난 2주간, 당신의 삶 전반에 대해 얼마나 자주 만족했습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 14 | 지난 2주간, 당신의 질병으로 좌절감을 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Physical function subscale of the Korean version of the Gastrointestinal Quality of Life Index
| Subscales | Question number | Korean version |
|---|---|---|
| Physical function (7 items) | 15 | 지난 2주간, 지치고 피곤함을 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| 16 | 지난 2주간, 몸이 불편한 것을 얼마나 자주 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 17 | 지난 1주간(7일), 밤에 자면서 한번이라도 깬 경험을 한 날이 몇 일입니까? ① 매일 밤 ② 5~6일 ③ 3~4일 ④ 1~2일 ⑤ 전혀 없음 |
|
| 18 | 당신의 질병으로 본인의 외모가 얼마나 볼품 없어졌다고 생각합니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| 19 | 당신의 질병으로 본인의 체력이 얼마나 떨어졌다고 생각합니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| 20 | 당신의 질병으로 본인의 기력(스테미나)는 얼마나 감소했다고 생각하십니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| 21 | 당신의 질병으로 본인의 건강상태가 얼마나 나빠졌다고 생각하십니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
Social function and medical treatment subscales of the Korean version of the Gastrointestinal Quality of Life Index
| Subscales | Question number | Korean version |
|---|---|---|
| Social function (4 items) | 22 |
지난 2주간, 직장, 학업, 집안일 등 정상적인 일상 활동을 유지할 수 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
| 23 |
지난 2주간, 운동이나 취미생활 등 정상적인 여가 활동을 유지할 수 있었습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
|
| 25 | 당신의 질병이 가까운 사람들과의 관계를 얼마나 많이 바꿨습니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| 26 | 당신의 질병이 본인의 성생활에 얼마나 나쁜 영향을 미쳤습니까? ① 아주 많이 ② 많이 ③ 다소 ④ 약간 ⑤ 전혀 아님 |
|
| Medical treatment (1 item) | 24 | 지난 2주간, 치료로 인해 구속감을 느꼈습니까? ① 항상 ② 대부분 ③ 때때로 ④ 드물게 ⑤ 전혀 없음 |
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Results of the pre-test for the cognitive interview
| Variable | Total patients (n=20) |
|---|---|
| Baseline characteristics | |
| Age (yr) | 61.8 (44~82) |
| Sex | |
| Male | 16 (80) |
| Female | 4 (20) |
| GIQLI | |
| Total score | 104 (93.0~111.2) |
| Symptoms | 63 (53.7~67.5) |
| Emotions | 13 (11.0~14.2) |
| Physical function | 17 (14.5~21.5) |
| Social function | 9 (7.0~11.0) |
| Medical treatment | 3 (2.0~4.0) |
| Interview | |
| Question 1 | 6.5 (5.0~10.0) |
| Question 2 | 4 (3.0~4.0) |
| Question 3 | 4 (4.0~4.0) |
Values are presented as median (interquartile range) or number (%).
Question 1 = “How long did you take to complete the questionnaire?”. Question 2 = “Are the meanings of the questions clear and unambiguous?”. Question 3= “ Are the questions in the questionnaire adequately described in terms of expressions used in daily life?”.
GIQLI = Gastrointestinal Quality of Life Index.
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Please note that the questions shown in “a” are inverted scores and should be carefully summed.
Values are presented as median (interquartile range) or number (%). Question 1 = “How long did you take to complete the questionnaire?”. Question 2 = “Are the meanings of the questions clear and unambiguous?”. Question 3= “ Are the questions in the questionnaire adequately described in terms of expressions used in daily life?”. GIQLI = Gastrointestinal Quality of Life Index.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN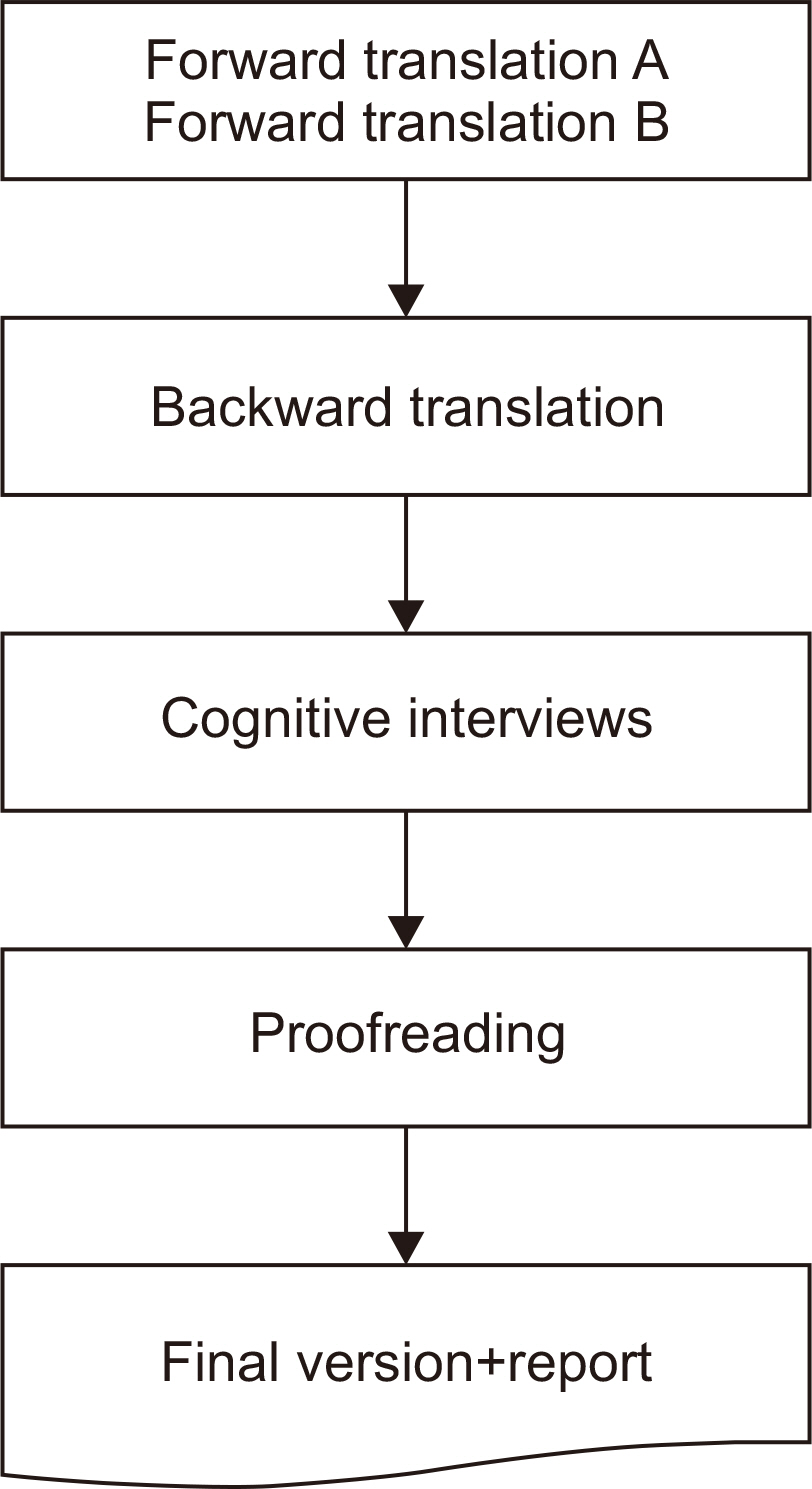
 Cite
Cite

