Abstract
-
Purpose
The aim of this study is to demonstrate clinical characteristics of refeeding syndrome (RS) and clinical utility of several guidelines including American Society for Parenteral and Enteral Nutrition consensus recommendations for RS and National Institute for Clinical Excellence guidelines.
-
Materials and Methods
Eighty-six patients screened for RS based on two guidelines were enrolled in this study. We evaluated the severity of RS after the initiation of ‘dextrose infusion’ and ‘balanced nutrition support’ with calculation of 5-day electrolyte changes. The primary outcome was 6-month mortality and secondary outcomes were duration of intensive care unit stay in days, duration of mechanical ventilation in days, and ventilator-free days at the 28th day.
-
Results
We observed statistically different distributions in terms of prevalence of RS on the basis of two different start times of caloric support (P=0.021). There was no statistically significant relationship between the risk of RS and severity of RS based on both guidelines. Also, the relevance between severity of RS and outcomes was not significant in our study. In multivariable logistic regression analysis of factors associated with outcomes, the significant factor for primary outcome was the ‘patients with significant risk’ (odds ratio, 9.65; 95% confidence interval, 1.83~50.90; P=0.007).
-
Conclusion
We demonstrated that even initial administration of dextrose solution and propofol could cause severe RS in critically ill patients. In addition, significant risk of RS was a predictive factor for 6-month mortality. Thus, it is essential to monitor the occurrence of RS even during initial resuscitation in patients with unstable metabolism.
-
Keywords: Intensive care unit; Mortality; Phosphorus; Potassium; Refeeding syndrome
INTRODUCTION
Critically ill patients are in a potentially fatal condition with poor hemostasis due to infection, surgical procedures, trauma, and other factors causing metabolic imbalance. Conditions can stabilize with improvement in organ function after appropriate resuscitation and intensive treatment. Nutritional support, which is inevitable for patient survival, should be performed carefully according to one’s metabolic status [
1]. It is very hard for clinicians to initiate nutritional support and establish the appropriate amount and route of supplementation while continuously monitoring change in metabolic status [
2,
3]. Excessive and inappropriate feeding could cause various kinds of problems such as gastrointestinal symptoms including constipation or diarrhea, liver toxicity related to parenteral nutritional support, catheter-related infection, and high caloric intake-related complications including hyperglycemia, and refeeding syndrome (RS).
RS is a serious and potentially fatal condition that can occur while refeeding or increasing caloric intake, especially in malnourished patients [
4,
5]. RS is caused by a sudden shift in the electrolytes that help the patient’s body metabolize food and this unstable metabolism may induce organ failure. However, clinicians quite often overlook the occurrence of RS especially in critically ill patients due to a wide range of clinical symptoms from electrolyte imbalance, nausea and lethargy to respiratory insufficiency, cardiac failure and death. We should concentrate on preventing the occurrence of RS during intensive care while treating high-risk patients to decrease mortality rate [
2,
4,
6,
7].
According to the “ASPEN consensus recommendations for refeeding syndrome”, high-risky patients for RS include those with anorexia nervosa, mental health disorders, alcohol or substance-use disorders, athletes, critically-ill patients and those who got cancer treatment or renal replacement therapy [
8-
11]. To prevent the occurrence of RS and improve outcomes of critical care, clinicians should closely monitor changes in electrolytes and sufficiently replace them [
2,
3,
11].
Historically RS was clinically diagnosed by hypophosphatemia and organ dysfunction [
4,
12,
13]. Many research groups such as the National Institute for Clinical Excellence (NICE), the Irish Society for Clinical Nutrition & Metabolism, and the Comprehensive Nutrition Survey in Gujarat previously suggested the risk factors for RS, devised diagnostic criteria for RS, and also recommended feeding protocols for avoiding RS [
1,
3,
14]. Friedli et al. [
15] did systemic review in 2017 and concluded that there is lack of consensus for the definition of RS and therefore incidences of RS showed wide heterogeneity. This group also suggested more detailed risk classification based on the NICE guidelines presented in 2006 and developed a new monitoring algorithm and treatment protocols [
16]. Despite all these trials for identification of methods for diagnosis and treatment of RS, most previous studies were conducted on anorexia patients, not critically ill patients, and so there are no applicable diagnostic criteria and feeding protocols only for critically ill patients. The reality is that each medical center treats patients according to their own protocols.
Recently a new guideline for RS was proposed by the American Society for Parenteral and Enteral Nutrition (ASPEN) in 2020, the “ASPEN consensus recommendations for refeeding syndrome” [
11]. These recommendations were produced based on a majority of previous studies associated with RS and various prospective and retrospective studies for critically-ill patients within 5 years. New criteria for risk evaluation, diagnostic classification and feeding protocol were introduced [
11]. Risk evaluation was determined by six total risk factors including body mass index (BMI), weight loss, caloric intake, abnormal prefeeding potassium, phosphorus, or magnesium serum concentrations, loss of subcutaneous fat, loss of muscle mass, and higher-risk comorbidities (
Supplementary Table 1) [
11]. Diagnostic criteria included a decrease in phosphorus, potassium and magnesium and categorized decreases of three kinds of electrolytes. These were used to describe a new classification of the severity of RS (mild [10%~20%], moderate [20%~30%], severe [>30%]) (
Supplementary Table 2). In terms of the feeding protocol proposed by ASPEN recommendations, it was notable that they emphasized not just total caloric intake, but also the amount of dextrose at the time of initiating nutrition support, while previous studies only focused on the amount of balanced nourishment and how to advance it [
2,
3,
11,
14]. We focused on this recommendation because we previously experienced many cases associated with RS right after administration of dextrose-contained fluids at the start of resuscitation to treat shock patients in our center. Thus, based on our clinical experiences, we have been trying to supplement the deficient electrolytes at the time of admission at intensive care unit (ICU) to prevent the occurrence of RS.
The aim of this study is to demonstrate clinical characteristics of RS and identify the clinical utility of several guidelines of RS including ASPEN consensus recommendations for RS and NICE guidelines.
MATERIALS AND METHODS
1. Objectives
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB no. 20211738) and written informed consent was obtained from each patient and guardian to use data for statistical analysis. We set up four goals to analyze in this study. First of all, we compared the prevalence of RS based at the start of “dextrose infusion” and “balanced nutrition support”, which was defined as enteral feeding or parenteral feeding with formulas containing carbohydrates, lipids, and proteins properly. We sought to verify if administration of crystalloid fluids and sedatives (especially propofol) before balanced nutrition support influences the occurrence of RS. Second, we tried to clarify if this was relevant for either risk of RS and severity of RS based on ASPEN and NICE guidelines. Third, we sought to compare the outcomes between each group categorized by diagnostic criteria for RS included in ASPEN guidelines. Lastly, we analyzed the factors associated with outcomes.
2. Study design and patients
Among 456 critically ill patients who had been admitted to the surgical ICU in Asan Medical Center, Seoul, Korea between June 2020 and January 2021, 121 patients who stayed more than 72 hours at ICU were screened for RS based on both two guidelines: the ASPEN guidelines and NICE guidelines. Thus, those patients were subjected to a risk evaluation based on both “ASPEN consensus criteria for identifying adult patients at risk for refeeding syndrome” (
Supplementary Table 1) and “Criteria for determining people at high risk of developing refeeding problems” suggested by NICE guidelines (
Supplementary Table 3). Risk of RS was categorized as ‘without risk’, ‘moderate risk’ and ‘significant risk’ based on the ASPEN guidelines, and as ‘without risk’ and ‘with risk’ based on the NICE guideline (
Supplementary Tables 1, 3). Patients who got renal replacement therapy and had been re-admitted to ICU in the observational period were excluded. Overall, 86 patients were enrolled in this study (
Fig. 1).
Baseline characteristics of enrolled patients were investigated: age, sex, BMI, route of ICU admission, admission type, cause of ICU admission, diagnosis, Acute Physiology and Chronic Health Evaluation-IV (APACHE-IV) score, underlying disease, and whether a ventilator, or Extracorporeal Membrane Oxygenation (ECMO) were used.
In the process of risk evaluation, BMI, weight loss, caloric intake, serum electrolyte concentrations (especially potassium, phosphorus, or magnesium), loss of subcutaneous fat, loss of muscle mass, and higher-risk comorbidities were evaluated as nutrient factors. BMI was calculated with weight and height at the time of admission to the ICU. The information associated with weight loss, history of alcohol or drug abuse, and caloric intake was obtained for a set period of time like a week, a month, or 3 month through guardian interviews. Electrolyte levels were evaluated right before resuscitation began. Hypophosphatemia was defined as less than 2.5 mg/dL, hypokalemia was defined as less than 3.5 mmol/L, hypomagnesemia was defined as less than 1.8 mg/dL. Higher-risk comorbidities were assessed with in-hospital medical records and additional guardian interviews, if necessary. References associated with evaluation of loss of subcutaneous fat and muscle mass were quite ambiguous, so we used the records from our Nutritional Support Team.
Prior to the comparison of the prevalence of RS based on two different times of beginning caloric support (the first objective of this study), we first investigated the daily electrolyte levels for 5 days after the initiation of ‘dextrose infusion’ and ‘balanced nutrition support’. Then, we calculated the electrolyte change from the initial value to the minimum value within 5 days and described it as a ratio. Using these calculated values, based on “Diagnostic criteria for refeeding syndrome” suggested by ASPEN guidelines (
Supplementary Table 2), we categorized those finally enrolled patients into four groups: ‘no RS’ group, ‘mild RS’ group, ‘moderate RS’ group, and ‘severe RS’ group (20% included in moderate RS, and 30% included in severe RS) on the basis of two different start times of caloric support. All kinds of subsequent analyses were conducted with severity classification into four categories based on the initiation of ‘dextrose infusion’.
The primary outcome was 6-month mortality. Secondary outcomes were duration of ICU stay in days, duration of mechanical ventilation in days, and ventilator-free days at 28th days from admission. Expiration date was confirmed by the reports if that patient died during critical care at our center, and if not, we checked the insurance expiration date.
5. Statistical analysis
Descriptive data for patient characteristics were calculated for all variables. Data normality was assessed by visual inspection of the distribution. A marginal homogeneity test was conducted while describing the comparison of prevalence of RS between two groups, assessed based on the time of beginning ‘dextrose infusion’ and ‘balanced nutrition support’. After describing some baseline characteristics and confirming the relevance between risk and severity of RS, categorical variables were presented as frequency or percentage and analysis of variance, and a chi-square test or Fisher’s exact test was conducted. Continuous data are reported as mean±standard deviation or as median (and interquartile range) depending on data distribution and a Kruskal–Wallis test was performed to compare between four groups of RS severity. Levene’s test was performed to assess the equality of variances.
Relevance between outcomes and severity of RS was also analyzed by Fisher’s exact test and the Kruskal–Wallis test. Univariable and multivariable logistic regression analyses were performed to find factors for primary outcome and secondary outcomes. Odds ratios (ORs) and corresponding 95% confidence interval (CI) were estimated using both the univariate effect and multivariate effect. Variables considered were age, sex, BMI, APACHE-IV score, risk of RS, and severity of RS, which was newly classified into severe and non-severe groups. Data analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Statistical significances of all analyses were evaluated based on a P-value less than 0.05.
RESULTS
1. Baseline characteristics
A total of 86 critically-ill patients eligible for this study were enrolled in analysis. Baseline characteristics of enrolled patients are described according to the severity of RS in
Table 1. The mean age of patients was 68.27±13.13, and the mean BMI was 23.56±4.21. Patients with BMI values of 18.5 or higher accounted for 93.0%, those with BMI values of 16.0 to 18.5 accounted for 7.0%. Analysis of the route of ICU admission showed that 8.1% of enrolled patients were admitted through the emergency room before critical care, 17.4% were hospitalized at the general ward, 44.2% were transferred from other ICUs, and 30.2% were treated surgically before admission to the ICU. In terms of admission type, the patients who received elective surgery accounted for 24.4%, those who received an emergency operation accounted for 33.7%, and the remaining 41.8% were treated intensive care without surgical procedure. 52.3% of patients were admitted to the ICU for postoperative monitoring, 24.4% had respiratory failure, and 9.3% were in septic shock. In total, 58.1% of patients were treated critical care due to malignant disease, and 38.4% previously received cancer treatment. The incidence of diabetes mellitus and hypertension was 32.6% and 23.3%, respectively. Mean APACHE-IV score was 71.40±18.85; 84% of total patients were treated using mechanical ventilation and only 1% needed ECMO therapy. No statistical difference between each group was confirmed for all parameters including BMI except underlying tumorous conditions (even if it was malignant or not) (P-value <0.05).
Analysis of the occurrence of RS assessed from two different instances of caloric support is shown in
Table 2. As shown, 77 of 86 patients developed RS with the initiation of ‘dextrose infusion’: mild 16 (18.6%), moderate 15 (17.4%), and severe 46 (53.5%). In contrast, 67 patients developed RS with ‘balanced nutrition support’: mild 21 (24.4%), moderate 18 (20.9%), and severe 28 (32.6%). There was statistical difference associated with prevalence of RS (P=0.021). More patients with severe RS were observed even with dextrose infusion for resuscitation than with balanced nutritional support. Based on this result of our first objective in this study, we decided to apply severity classification into four categories based on the initiation of ‘dextrose infusion’ to all subsequent analyses.
The result of the analysis of relevance between risk of RS assessed by both “ASPEN criteria for identifying adult patients at risk for refeeding syndrome” and NICE guidelines and severity of RS classified by “Diagnostic criteria for refeeding syndrome” are shown in
Table 3. Based on the ASPEN guideline, 30 patients had no risk for RS (34.9%), 41 had moderate risk (47.7%), 15 had significant risk (17.5%), while the moderate risk group was the largest one among total patients. Among the moderate risk group, eight patients showed moderate RS (19.5%) and 20 patients had severe RS (48.8%). Among significant risk groups, four patients showed mild RS (26.7%), three patients had moderate RS (20.0%), and eight patients had severe RS (53.3%). There turned out to be no statistical correlation between risk and severity of RS (P=0.211), because among the patient group without risk, as many as 18 (60.0%) of those patients were diagnosed with severe RS. Based on NICE guidelines, 12 patients had no risk of RS (13.95%) and 74 were at risk of RS (86.05%). Among the patients who had risk of RS, 13 patients had mild RS (17.6%), 14 patients showed moderate RS (18.9%), and 39 had severe RS (52.7%). However, among the patient group without risk, seven patients (58.3%) were diagnosed severe RS, so no statistical correlation was identified (P=0.842).
Table 4 shows the relevance between severity of RS and clinical outcomes. However, there was no significant difference in mortality (P=0.711), length of ICU stay (P=0.472), duration of mechanical ventilation (P=0.576), or ventilator-free days at the 28th day (P=0.458) among groups.
Predictive factors associated with 6-month mortality were assessed by both univariable and multivariable logistic regression analysis as shown in
Table 5. For this analysis, patients were classified again into the ‘Severe RS group’ and ‘Non-severe RS group’. BMI and patient with significant risk were statistically significant factors as OR values were 0.85 (95% CI, 0.73~0.99; P=0.040) and 13.5 (95% CI, 2.78~65.40; P=0.001), respectively. In multivariable analysis with BMI and risk of RS, patients with moderate risk showed an OR of 3.68 (95% CI, 0.90~15.03; P=0.089) and patients with significant risk showed an OR of 9.65 (95% CI, 1.83~50.90; P=0.007).
DISCUSSION
In this study, we evaluated the prevalence and clinical meaning of RS in critically ill patients, and also investigated the relevance of known-high risk groups and the occurrence of RS. Most of all, we assessed two different times when RS developed in the ICU, applying the newest diagnostic criteria suggested by the “ASPEN consensus recommendations for refeeding syndrome”, that is, from ‘dextrose infusion’ and from ‘balanced nutrition support’. According to this newest guideline, the decreased amount of electrolytes was categorized as 10%, 20%, or 30% changes from the initial value, and these were described as mild RS, moderate RS, or severe RS, respectively. In our study, overall prevalence of RS was as much as 89.6% and 78.0% as assessed from the two different start times, respectively. Contrary to the various kinds of previous reports from other centers, there were much greater proportion of patients with RS when the decrease of electrolytes was evaluated as not just below a certain value, and the rate of electrolyte changes. Prevalence of RS assessed in our study itself had great significance in that most of patients initially treated in the ICU can be classified as those with RS. They were also categorized based on severity, so they could be treated with adequate replenishment of electrolytes even during the initial phase of critical care. Also, this study showed that electrolyte imbalance had already developed in early resuscitation periods because we started supplementation of nutrients unintentionally (i.e., not for the purpose of nutrition support), but also for drug delivery, and also administered propofol as a sedative at the time of ‘dextrose infusion’. The most valuable point of this research is that it is essential for clinicians to closely monitor RS not just at the time of initiation of nutrition support, but even at the early stage of resuscitation [
17-
19].
In addition to keeping a close watch on risky patients during intensive care, early recognition of patients at risk was also very important. Friedli et al. [
16] designed new guidelines for evaluation of risk of RS at 2018. The patient populations were divided as four groups: no risk, low risk, high risk and very high risk groups. There were a few studies explaining the relevance between this risk evaluation and outcomes of patients based on this classification as mentioned above, but there was no notable result reporting a relevance between risk assessment and actual incidence of RS [
20]. In this study, “ASPEN consensus recommendations for refeeding syndrome”, which specified criteria to identify the patients at risk for RS in advance was used as a risk evaluation. However, the analysis showed no significant relationship between the patients at risk and the development of RS in critically ill patients. This is because critically ill patients have too many reasons causing electrolyte imbalance including lots of fluids from resuscitation, various drugs, organ dysfunction, and/or combined comorbidities. Identifying RS in critically ill patients might be a very complicated process.
Previously, several retrospective studies reported the outcomes of RS diagnosed patients. Friedli et al. [
21] performed secondary analysis of randomized trial with RS confirmed patients and patients for which RS was not confirmed. They revealed that patients with RS had a significantly increased 6-month mortality rate (adjusted OR, 1.53 [95% CI, 1.02~2.29]; P-value <0.05) and also increased risk of ICU admission (adjusted OR, 2.71; P-value <0.05) [
21]. Olthof et al. [
19] compared the outcomes between patients with RS and patients without RS, and they concluded there was no significant result, which agrees with our study. Among neurocritically ill patients, Xiong et al. [
22] showed that RS is not rare in those patients and RS itself is an independent risk factor for 6-month mortality treating patients with brain damage. Bioletto et al. [
23] did a meta-analysis in 2021 to identify the relationship between RS and clinical outcomes. They insisted that patients with RS had increased 6-month mortality rate, but there was no difference in 1-month mortality rate due to intensive resuscitation and treatment. In our study, the primary outcome of 6-month mortality and secondary outcomes of total length of ICU stay, duration of mechanical ventilation, and ventilator-free days at 28th day in the ICU all appeared not to be related to the severity of RS. However, in an analysis of predictive factors for 6-month mortality, the risk of RS was shown as a significant factor, and it was especially significant if the RS risk group was 9.6 times riskier than the ‘no risk’ group. In terms of secondary outcomes, we could not find significant factors that prolonged ICU stay and aggravated respiratory function. A more finely controlled analysis with larger patient populations is necessary.
This study had several limitations. First of all, population size was too small for statistical analysis. Especially, logistic regression models were statistically unstable with a small number of patients suffering death within 6 months. Second, other confounders such as the use of steroids or diuretics, which could affect electrolyte level, were not finely controlled in this study. Third, there was no appropriate tool for defining organ dysfunction, so there might be selection bias in the severe RS group. Fourth, thiamine deficiency was not surveyed due to a lack of data in patient reports [
24].
CONCLUSION
In this study, we confirmed that even administration of crystalloid fluids including dextrose and propofol as sedatives could cause RS in critically ill patients. In addition, significant risk of RS was demonstrated as a predictive factor for poorer outcomes in terms of 6-month mortality. We should identify patients at risk for RS in advance and closely monitor electrolyte levels. Further study is necessary to determine how best to deal with RS in critically ill patients.
SUPPLEMENTARY MATERIALS
AUTHOR CONTRIBUTIONS
Conceptualization: SWH, SKH. Data curation: SWH, SKH. Formal analysis: SWH, SKH. Investigation: SWH, SKH. Methodology: SWH, SKH. Project administration: SWH, SKH. Resources: SWH, SKH. Software: SWH, SKH. Supervision: SWH, SKH. Validation: SWH, SKH. Visualization: SWH, SKH. Writing – original draft: SWH, SKH. Writing – review & editing: SWH, SKH.
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
FUNDING
None.
Fig. 1
Patient selection flow.
SICU = surgical intensive care unit; ICU = intensive care unit.
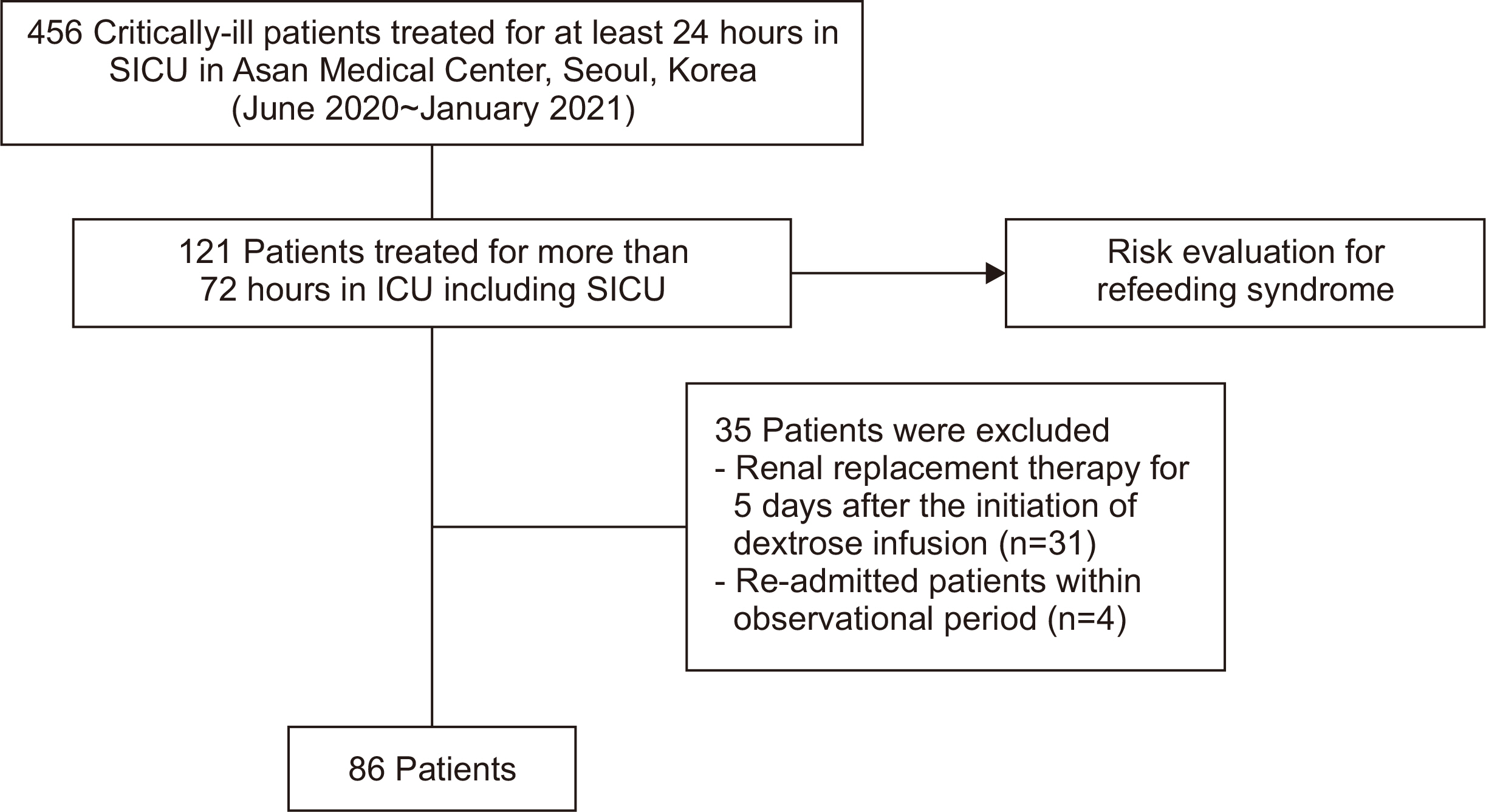
Table 1
|
Characteristic |
Total (n=86) |
No RS (n=9) |
Mild RS (n=16) |
Moderate RS (n=15) |
Severe RS (n=46) |
P-value |
|
Age (yr) |
68.27±13.13 |
68.33±11.35 |
66.50±13.67 |
64.20±20.85 |
70.21±9.72 |
0.595 |
|
Sex |
|
|
|
|
|
0.539 |
|
Male |
60 (69.8) |
8 (88.9) |
11 (68.7) |
9 (60.0) |
32 (69.6) |
|
|
Female |
26 (30.2) |
1 (11.1) |
5 (31.3) |
6 (40.0) |
14 (30.4) |
|
|
BMI (kg/m2) |
23.56±4.21 |
25.33±3.79 |
22.51±3.21 |
23.20±3.15 |
23.69±4.82 |
0.439 |
|
BMI ≥18.5 |
80 (93.0) |
9 (100) |
14 (87.5) |
14 (93.3) |
43 (93.5) |
0.847 |
|
BMI 16.0~18.5 |
6 (7.0) |
0 (0) |
2 (12.5) |
1 (6.7) |
3 (6.5) |
|
|
BMI <16.0 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
|
Route of ICU admission |
|
|
|
|
|
0.524 |
|
Emergency room |
7 (8.1) |
0 (0) |
2 (12.5) |
0 (0) |
5 (10.9) |
|
|
General ward |
15 (17.4) |
4 (44.4) |
3 (18.8) |
2 (13.3) |
6 (13.0) |
|
|
Transfer from other ICU |
38 (44.2) |
2 (22.2) |
6 (37.5) |
9 (60.0) |
21 (45.7) |
|
|
Operating room |
26 (30.2) |
3 (33.3) |
5 (31.3) |
4 (26.7) |
14 (30.4) |
|
|
Admission type |
|
|
|
|
|
0.117 |
|
Emergency operation |
29 (33.7) |
4 (44.4) |
4 (25.0) |
2 (13.3) |
19 (41.3) |
|
|
Elective surgery |
21 (24.4) |
0 (0) |
3 (18.8) |
5 (33.3) |
13 (28.3) |
|
|
Critical care without surgery |
36 (41.9) |
5 (55.6) |
9 (56.2) |
8 (53.3) |
14 (30.4) |
|
|
Cause of ICU admission |
|
|
|
|
|
0.167 |
|
Postoperative monitoring |
45 (52.3) |
2 (22.2) |
7 (43.8) |
7 (46.7) |
29 (63.0) |
|
|
Trauma |
5 (5.8) |
1 (11.1) |
1 (6.2) |
2 (13.3) |
1 (2.2) |
|
|
Septic shock |
8 (9.3) |
0 (0) |
2 (12.5) |
2 (13.3) |
4 (8.7) |
|
|
Hypovolemic shock |
4 (4.6) |
1 (11.1) |
0 (0) |
0 (0) |
3 (6.5) |
|
|
Respiratory failure |
21 (24.4) |
5 (55.6) |
6 (37.5) |
3 (20.0) |
7 (15.2) |
|
|
Hepatic failure |
1 (1.2) |
0 (0) |
0 (0) |
0 (0) |
1 (2.2) |
|
|
CNS change |
1 (1.2) |
0 (0) |
0 (0) |
0 (0) |
1 (2.2) |
|
|
Others |
1 (1.2) |
0 (0) |
0 (0) |
1 (6.7) |
0 (0) |
|
|
Diagnosis |
|
|
|
|
|
0.808 |
|
Malignancy |
50 (58.1) |
6 (66.7) |
10 (62.5) |
9 (60.0) |
25 (54.3) |
|
|
Bowel complication |
17 (19.8) |
1 (11.1) |
3 (18.8) |
2 (13.3) |
11 (23.9) |
|
|
AAA |
3 (3.5) |
0 (0) |
0 (0) |
1 (6.7) |
2 (4.3) |
|
|
Transplantation |
2 (2.3) |
0 (0) |
1 (6.2) |
0 (0) |
1 (2.2) |
|
|
Trauma |
6 (7.0) |
2 (22.2) |
0 (0) |
2 (13.3) |
2 (4.3) |
|
|
Others |
8 (9.3) |
0 (0) |
2 (12.5) |
1 (6.7) |
5 (10.9) |
|
|
APACHE-IV score |
71.40±18.85 |
75.66±19.41 |
68.87±16.55 |
62.00±24.48 |
74.52±16.76 |
0.123 |
|
Underlying disease |
|
|
|
|
|
|
|
DM |
28 (32.6) |
4 (44.4) |
5 (31.3) |
4 (26.7) |
15 (32.6) |
0.840 |
|
HTN |
20 (23.3) |
5 (55.6) |
3 (18.8) |
1 (6.7) |
11 (23.9) |
0.066 |
|
Heart disease |
6 (7.0) |
0 (0) |
1 (6.3) |
0 (0) |
5 (10.9) |
0.637 |
|
Respiratory disease |
5 (5.8) |
0 (0) |
0 (0) |
0 (0) |
5 (10.9) |
0.320 |
|
Renal disease |
6 (7.0) |
1 (11.1) |
2 (12.5) |
2 (13.3) |
1 (2.2) |
0.126 |
|
Liver disease |
5 (5.8) |
1 (11.1) |
1 (6.3) |
0 (0) |
3 (6.5) |
0.685 |
|
Neurological disease |
13 (15.1) |
1 (11.1) |
1 (6.3) |
1 (6.7) |
10 (21.7) |
0.422 |
|
Other tumorous condition |
33 (38.4) |
5 (55.6) |
10 (62.5) |
6 (40.0) |
12 (26.1) |
0.045 |
|
Transplant |
1 (1.2) |
0 (0) |
1 (6.3) |
0 (0) |
0 (0) |
0.465 |
|
Others |
6 (7.0) |
1 (11.1) |
1 (6.3) |
2 (13.3) |
2 (4.3) |
0.398 |
|
Ventilator application |
84 (97.7) |
9 (100) |
15 (93.8) |
15 (100) |
45 (97.8) |
0.716 |
|
ECMO application |
1 (1.2) |
0 (0) |
0 (0) |
0 (0) |
1 (2.2) |
>0.999 |
Table 2Prevalence of RS assessed from the beginning of ‘dextrose infusion’ and ‘balanced nutrition support’
|
Severity |
Dextrose infusion (n=86) |
Balanced nutrition support (n=86) |
P-value |
|
No RS |
9 (10.5) |
19 (22.1) |
0.021 |
|
Mild RS |
16 (18.6) |
21 (24.4) |
|
|
Moderate RS |
15 (17.4) |
18 (20.9) |
|
|
Severe RS |
46 (53.5) |
28 (32.6) |
|
Table 3Relevance between risk of RS and severity of RS based on both ASPEN and NICE guidelines (between each group)
|
Risk |
Refeeding syndrome |
P-value |
|
|
None (n=9) |
Mild RS (n=16) |
Moderate (n=15) |
Severe (n=46) |
|
ASPEN guidelines |
|
|
|
|
0.211 |
|
Without RS risk |
1 (3.3) |
7 (23.3) |
4 (13.3) |
18 (60.0) |
|
|
Moderate Risk |
8 (19.5) |
5 (12.2) |
8 (19.5) |
20 (48.8) |
|
|
Significant Risk |
0 (0) |
4 (26.7) |
3 (20.0) |
8 (53.3) |
|
|
NICE guidelines |
|
|
|
|
0.842 |
|
Without RS risk |
1 (8.3) |
3 (25.0) |
1 (8.3) |
7 (58.3) |
|
|
With RS risk |
8 (10.8) |
13 (17.6) |
14 (18.9) |
39 (52.7) |
|
Table 4Clinical outcomes according to the severity of RS
|
Clinical outcomes |
Refeeding syndrome |
P-value |
|
|
No RS (n=9) |
Mild RS (n=16) |
Moderate RS (n=15) |
Severe RS (n=46) |
|
Primary outcome |
|
|
|
|
|
|
6-month mortality |
2 (22.2) |
3 (18.8) |
3 (20.0) |
15 (32.6) |
0.711 |
|
Secondary outcomes |
|
|
|
|
|
|
Length of ICU stay |
18 (10~37) |
14 (8~30) |
10 (6~22) |
12 (9~25) |
0.472 |
|
Duration of MV |
13 (8~40) |
13 (7~28) |
9 (4~22) |
11 (7~26) |
0.576 |
|
VFDs at 28th day |
15 (0~20) |
15 (3~22) |
19 (6~24) |
17 (2~21) |
0.458 |
Table 5Predictive factors associated with 6-month mortality by logistic regression analysis
|
Factor |
Primary outcome (6-month mortality) |
|
|
Univariable |
|
Multivariable |
|
|
|
OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
|
Age |
1.00 (0.97~1.04) |
0.729 |
|
|
|
|
Sexa
|
1.01 (0.36~2.87) |
0.980 |
|
|
|
|
BMI |
0.85 (0.73~0.99) |
0.040 |
|
0.90 (0.76~1.07) |
0.265 |
|
APACHE-IV score |
1.01 (0.98~1.03) |
0.486 |
|
|
|
|
RS Risk: moderate |
3.30 (0.83~13.09) |
0.089 |
|
3.68 (0.90~15.03) |
0.089 |
|
RS Risk: significant |
13.50 (2.78~65.40) |
0.001 |
|
9.65 (1.83~50.90) |
0.007 |
|
Severe RS |
1.93 (0.72~5.20) |
0.191 |
|
|
|
References
- 1. National Collaborating Centre for Acute Care. 2006. Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition [Internet]. National Collaborating Centre for Acute Care; London: Available from: https://www.nice.org.uk/guidance/cg32. [cited 2021 Nov 25
- 2. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. ArticlePubMed
- 3. Boland K, Solanki D, O'Hanlon C. Prevention and treatment of refeeding syndrome in the acute care setting. Dublin: Irish Society for Clinical Nutrition and Metabolism; 2013.
- 4. Stanga Z, Brunner A, Leuenberger M, Grimble RF, Shenkin A, Allison SP, et al. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr 2008;62:687-94. ArticlePubMedPDF
- 5. Hammami S, Aref HL, Khalfa M, Kochtalli I, Hammami M. Refeeding syndrome in adults with celiac crisis: a case report. J Med Case Rep 2018;12:22.ArticlePubMedPMCPDF
- 6. Kraft MD, Btaiche IF, Sacks GS. Review of the refeeding syndrome. Nutr Clin Pract 2005;20:625-33. ArticlePubMedPDF
- 7. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 2008;336:1495-8. ArticlePubMedPMC
- 8. Marinella MA. Refeeding syndrome: an important aspect of supportive oncology. J Support Oncol 2009;7:11-6.PubMed
- 9. Vignaud M, Constantin JM, Ruivard M, Villemeyre-Plane M, Futier E, Bazin JE, et al. Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: an observational study. Crit Care 2010;14:R172.ArticlePubMedPMCPDF
- 10. Silk Z, Jones L, Heath D. Refeeding syndrome: an important complication after bariatric surgery. Surg Obes Relat Dis 2011;7:e21-3. ArticlePubMed
- 11. da Silva JSV, Seres DS, Sabino K, Adams SC, Berdahl GJ, Citty SW, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract 2020;35:178-95; Erratum in: Nutr Clin Pract 2020;35:584-5. ArticlePubMedPDF
- 12. Coşkun R, Gündoğan K, Baldane S, Güven M, Sungur M. Refeeding hypophosphatemia: a potentially fatal danger in the intensive care unit. Turk J Med Sci 2014;44:369-74. ArticlePubMed
- 13. Srivastava S, Shen M, Bowman A, Seres D. Characterizing the clinical impact of refeeding syndrome: serum phosphorus decrement does not impact length of stay (P12-038-19). Curr Dev Nutr 2019;3(Suppl 1):nzz035.P12-038-19. ArticlePMC
- 14. Hamilton A, Allsopp K. CNSG East Cheshire NHS trust guidelines for prevention and management of refeeding syndrome in adults. Macclesfield: CNSG; 2018.
- 15. Friedli N, Stanga Z, Sobotka L, Culkin A, Kondrup J, Laviano A, et al. 2017;Revisiting the refeeding syndrome: results of a systematic review. Nutrition 35:151-60. ArticlePubMed
- 16. Friedli N, Stanga Z, Culkin A, Crook M, Laviano A, Sobotka L, et al. Management and prevention of refeeding syndrome in medical inpatients: an evidence-based and consensus-supported algorithm. Nutrition 2018;47:13-20. ArticlePubMed
- 17. Rio A, Whelan K, Goff L, Reidlinger DP, Smeeton N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: prospective cohort study. BMJ Open 2013;3:e002173. ArticlePubMedPMC
- 18. Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, et al. Refeeding Syndrome Trial Investigators Group. 2015;Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med 3:943-52. ArticlePubMed
- 19. Olthof LE, Koekkoek WACK, van Setten C, Kars JCN, van Blokland D, van Zanten ARH. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr 2018;37:1609-17. ArticlePubMed
- 20. Yoshida M, Izawa J, Wakatake H, Saito H, Kawabata C, Matsushima S, et al. Mortality associated with new risk classification of developing refeeding syndrome in critically ill patients: a cohort study. Clin Nutr 2021;40:1207-13. ArticlePubMed
- 21. Friedli N, Baumann J, Hummel R, Kloter M, Odermatt J, Fehr R, et al. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: secondary analysis of a randomized trial. Medicine (Baltimore) 2020;99:e18506. PubMedPMC
- 22. Xiong R, Huang H, Wu Y, Wang S, Wang D, Ji Z, et al. 2021;Incidence and outcome of refeeding syndrome in neurocritically ill patients. Clin Nutr 40:1071-6. ArticlePubMed
- 23. Bioletto F, Pellegrini M, Ponzo V, Cioffi I, De Francesco A, Ghigo E, et al. Impact of refeeding syndrome on short- and medium-term all-cause mortality: a systematic review and meta-analysis. Am J Med 2021;134:1009-18.e1. ArticlePubMed
- 24. Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care 2011;14:610-7. ArticlePubMed

 , Suk-Kyung Hong, M.D., Ph.D.2
, Suk-Kyung Hong, M.D., Ph.D.2


 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

