Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > J Clin Nutr > Volume 7(2); 2015 > Article
- Original Article Clinical Application of Bioelectrical Impedance Analysis and its Phase Angle for Nutritional Assessment of Critically III Patients
- Hyung-Sook Kim1, Eun Sook Lee1, Yeon Joo Lee2,3, Jae Ho Lee2, Choon-Taek Lee2, Young-Jae Cho2
- 중환자에서 영양상태 평가지표로서 생체전기 임피던스를 이용한 위상 각의 활용
- 김형숙1, 이은숙1, 이연주2,3, 이재호2, 이춘택2, 조영재2
-
Journal of the Korean Society for Parenteral and Enteral Nutrition 2015;7(2):54-61.
DOI: https://doi.org/10.15747/jcn.2015.7.2.54
Published online: August 31, 2015
Department of Pharmacy, Seoul National University Bundang Hospital, Seongnam, Korea
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
Interdepartment of Critical Care Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- Correspondence to Young-Jae Cho Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 463-707, Korea Tel: +82-31-787-7058, Fax: +82-31-787-4034, E-mail: lungdrcho@snubh.org
Copyright: © Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,797 Views
- 20 Download
- 3 Crossref
Abstract
-
Purpose: Phase angle (PA) is objectively determined from resistance and reactance measured by bioelectrical impedance analysis (BIA)−a quick, noninvasive method. The aim of this study was to evaluate the clinical application of PA by BIA for nutritional assessment of critically ill patients.
-
Methods: Eighty nine adult patients admitted to a medical intensive care unit (ICU) of a tertiary academic hospital from August 2012 to September 2013 were analyzed. PA values were measured by direct segmental multi-frequency BIA. As traditional nutrition assessment tools, body mass index (BMI), serum albumin levels, total lymphocyte counts, and our hospital’s nutrition screening index (NSI) were also recorded. Correlations between the results of BIA and other traditional parameters were analyzed.
-
Results: PA showed correlation with traditional nutritional parameters, including BMI (r=0.479), serum albumin (r=0.347), and NSI score (r=0.483). Patients with PA lower than the median value (3.5°) had significantly lower nutritional status, increased duration of mechanical ventilation (P=0.039), and increased length of ICU stay (P=0.041).
-
Conclusion: PA, as a reflection of body cell mass, measured by BIA could be a potentially useful parameter for nutritional assessment in critically ill patients.
INTRODUCTION
MATERIALS AND METHODS
| Coding | Albumin (in mg/dL) | TLC (mm3) | BMI (kg/m2) | Age (y) |
|---|---|---|---|---|
| 1 | <3.5 | <900 | <18.5 | >65 |
| 2 | ≥3.5 | ≥900 | ≥18.5 | ≤65 |
RESULTS
| Characteristic | Initially measured group |
|---|---|
| Age (y) | 65.2±14.5 |
| Sex (male/female) | 63/26 |
| APACHE II score | 20.1±9.53 |
| Nutritional variables | |
| NSI score | 9.3±1.5 |
| Malnourished according to NSI score | 36 (40.4) |
| Actual body weight (kg) | 59.1±11.4 |
| Body mass index (kg/m2) | 22.1±3.6 |
| Albumin (mg/dL) | 3.3±0.7 |
| Total lymphocyte count (mm3) | 1,240.1±905.8 |
| Phase angle (o) | 3.7±1.4 |
| Fat-free mass (kg) | 48.2±9.9 |
| Body cell mass (kg) | 30.3±6.6 |
| ICU admission diagnosis | |
| Respiratory | 47 (52.8) |
| Cardiovascular | 17 (19.1) |
| Sepsis | 15 (16.9) |
| Gastrointestinal | 4 (4.5) |
| Trauma | 1 (1.1) |
| Other | 5 (5.6) |
| Clinical variables | |
| Duration of mechanical ventilation (d)a | 6.4±12.2 |
| Length of ICU stay (d)a | 9.4±13.5 |
| PA ≤3.5× (n=44) | PA >3.5× (n=45) | P-value | |
|---|---|---|---|
| Age (y) | 70.3±3.7 | 60.2±13.7 | 0.001 |
| Sex (male/female) | 29/15 | 34/11 | 0.358 |
| APACHE II score | 22.7±10.5 | 17.5±7.8 | 0.010 |
| Nutritional variables | |||
| NSI score | 8.8±1.3 | 9.9±1.6 | <0.001 |
| Malnourished according to NSI score (%) | 19 (43.2) | 17 (37.8) | 0.669 |
| Phase angle (o) | 2.6±0.6 | 4.8±1.0 | <0.001 |
| Actual body weight (kg) | 55.0±9.8 | 63.0±11.5 | 0.001 |
| Body mass index (kg/m2) | 20.9±3.6 | 23.3±3.2 | 0.001 |
| Albumin (mg/dL) | 3.1±0.6 | 3.5±0.7 | 0.005 |
| Total lymphocyte count (mm3) | 1,076.0±743.9 | 1,400.6±1,023.1 | 0.090 |
| Fat-free mass (kg) | 44.8±8.2 | 51.6±10.3 | 0.001 |
| Clinical variables | |||
| ICU mortality (%) | 14 (31.8) | 9 (20.0) | 0.151 |
| Length of ICU stay (d)a | 9.9±9.7 | 5.9±5.6 | 0.041 |
| Duration of mechanical ventilation (d)a | 6.5±9.3 | 2.8±4.3 | 0.039 |
DISCUSSION
CONCLUSION
- 1. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33(3):277-316. ArticlePubMed
- 2. Jacobs DO. Use of bioelectrical impedance analysis measurements in the clinical management of critical illness. Am J Clin Nutr 1996;64(3 Suppl):498S-502S. ArticlePubMed
- 3. Kyle UG, Unger P, Dupertuis YM, Karsegard VL, Genton L, Pichard C. Body composition in 995 acutely ill or chronically ill patients at hospital admission: a controlled population study. J Am Diet Assoc 2002;102(7):944-55. ArticlePubMed
- 4. Thibault R, Pichard C. The evaluation of body composition: a useful tool for clinical practice. Ann Nutr Metab 2012;60(1):6-16. ArticlePubMedPDF
- 5. Thibault R, Genton L, Pichard C. Body composition: why, when and for who? Clin Nutr 2012;31(4):435-47. ArticlePubMed
- 6. Kyle UG, Piccoli A, Pichard C. Body composition measurements: interpretation finally made easy for clinical use. Curr Opin Clin Nutr Metab Care 2003;6(4):387-93. ArticlePubMed
- 7. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr 2004;23(6):1430-53. ArticlePubMed
- 8. Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, et al. Accuracy of direct segmental multifrequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 2011;30(5):610-5. ArticlePubMed
- 9. Oliveira CM, Kubrusly M, Mota RS, Silva CA, Choukroun G, Oliveira VN. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J Ren Nutr 2010;20(5):314-20. ArticlePubMed
- 10. Gunn SM, Halbert JA, Giles LC, Stepien JM, Miller MD, Crotty M. Bioelectrical phase angle values in a clinical sample of ambulatory rehabilitation patients. Dyn Med 2008;7:14.ArticlePubMedPMCPDF
- 11. Kim S, Kim S, Sohn C. Development of nutrition screening index for hospitalized patients. Korean J Community Nutr 2006;11(6):779-84.Article
- 12. Gibson AL, Holmes JC, Desautels RL, Edmonds LB, Nuudi L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in Hispanic, black, and white adults. Am J Clin Nutr 2008;87(2):332-8. ArticlePubMed
- 13. Wirth R, Volkert D, Rösler A, Sieber CC, Bauer JM. Bioelectric impedance phase angle is associated with hospital mortality of geriatric patients. Arch Gerontol Geriatr 2010;51(3):290-4. ArticlePubMed
- 14. Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009;35(10):1728-37. ArticlePubMedPDF
- 15. Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R N MC, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 2005;24(4):502-9. ArticlePubMed
- 16. Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med 2004;32(2):350-7. ArticlePubMed
- 17. Petros S, Engelmann L. Enteral nutrition delivery and energy expenditure in medical intensive care patients. Clin Nutr 2006;25(1):51-9. ArticlePubMed
- 18. Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31(6):854-61. ArticlePubMed
- 19. Kyle UG, Genton L, Pichard C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin Nutr 2013;32(2):294-9. ArticlePubMed
- 20. Schwenk A, Beisenherz A, Römer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr 2000;72(2):496-501. ArticlePubMed
- 21. Paiva SI, Borges LR, Halpern-Silveira D, Assunção MC, Barros AJ, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer 2010;19(2):187-92. ArticlePubMedPDF
- 22. Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr 2004;92(6):957-62. ArticlePubMed
- 23. Gupta D, Lis CG, Dahlk SL, King J, Vashi PG, Grutsch JF, et al. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr J 2008;7:19.ArticlePubMedPMCPDF
- 24. Berbigier MC, Pasinato VF, Rubin Bde A, Moraes RB, Perry ID. Bioelectrical impedance phase angle in septic patients admitted to intensive care units. Rev Bras Ter Intensiva 2013;25(1):25-31. ArticlePubMedPMC
- 25. Zdolsek HJ, Lindahl OA, Sjöberg F. Non-invasive assessment of fluid volume status in the interstitium after haemodialysis. Physiol Meas 2000;21(2):211-20. ArticlePubMed
- 26. Foster GD, Knox LS, Dempsey DT, Mullen JL. Caloric requirements in total parenteral nutrition. J Am Coll Nutr 1987;6(3):231-53. ArticlePubMed
- 27. Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest 2003;124(1):297-305. ArticlePubMed
- 28. Schwenk A, Ward LC, Elia M, Scott GM. Bioelectrical impedance analysis predicts outcome in patients with suspected bacteremia. Infection 1998;26(5):277-82. ArticlePubMedPDF
- 29. Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr 2010;92(3):612-9. ArticlePubMed
- 30. Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr 2006;30(4):309-16. ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

- Optimal Enteral Nutrition Support Preserved Muscle Mass in Critically Ill Children
Kantisa Sirianansopa, Chavisa Rassameehirun, Sirinuch Chomtho, Orapa Suteerojntrakool, Lalida Kongkiattikul, Eric Gumpricht
Journal of Nutrition and Metabolism.2022; 2022: 1. CrossRef - Prognostic value of phase angle and bioelectrical impedance vector in critically ill patients: A systematic review and meta-analysis of observational studies
Júlia Lima, Igor Eckert, Maria Cristina Gonzalez, Flávia Moraes Silva
Clinical Nutrition.2022; 41(12): 2801. CrossRef - Bioelectrical Impedance Analysis for Prediction of Early Complications after Gastrectomy in Elderly Patients with Gastric Cancer: the Phase Angle Measured Using Bioelectrical Impedance Analysis
Byunghyuk Yu, Ki Bum Park, Ji Yeon Park, Seung Soo Lee, Oh Kyoung Kwon, Ho Young Chung
Journal of Gastric Cancer.2019; 19(3): 278. CrossRef
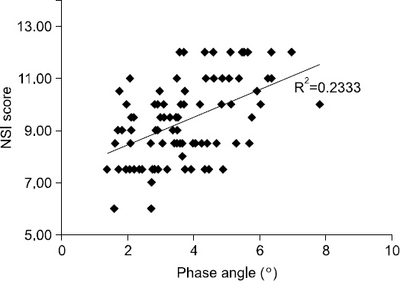
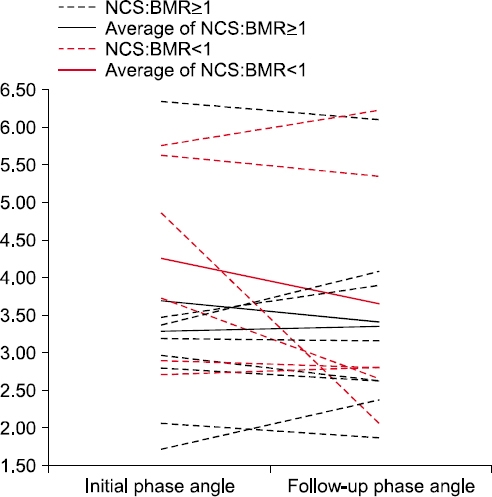
Fig. 1
Fig. 2
Coding of nutrition screeing index
| Coding | Albumin (in mg/dL) | TLC (mm3) | BMI (kg/m2) | Age (y) |
|---|---|---|---|---|
| 1 | <3.5 | <900 | <18.5 | >65 |
| 2 | ≥3.5 | ≥900 | ≥18.5 | ≤65 |
Clinical characteristics of subjects and nutritional functional variables: initially measured group (n=89)
| Characteristic | Initially measured group |
|---|---|
| Age (y) | 65.2±14.5 |
| Sex (male/female) | 63/26 |
| APACHE II score | 20.1±9.53 |
| Nutritional variables | |
| NSI score | 9.3±1.5 |
| Malnourished according to NSI score | 36 (40.4) |
| Actual body weight (kg) | 59.1±11.4 |
| Body mass index (kg/m2) | 22.1±3.6 |
| Albumin (mg/dL) | 3.3±0.7 |
| Total lymphocyte count (mm3) | 1,240.1±905.8 |
| Phase angle (o) | 3.7±1.4 |
| Fat-free mass (kg) | 48.2±9.9 |
| Body cell mass (kg) | 30.3±6.6 |
| ICU admission diagnosis | |
| Respiratory | 47 (52.8) |
| Cardiovascular | 17 (19.1) |
| Sepsis | 15 (16.9) |
| Gastrointestinal | 4 (4.5) |
| Trauma | 1 (1.1) |
| Other | 5 (5.6) |
| Clinical variables | |
| Duration of mechanical ventilation (d) |
6.4±12.2 |
| Length of ICU stay (d) |
9.4±13.5 |
aICU survival subgroup, n=66.
Correlations between phase angle (PA), body cell mass (BCM), and nutritional markers
| Nutritional marker | PA | BCM | ||
|---|---|---|---|---|
| r | P | r | P | |
| Actual body weight | 0.509 | <0.001 | 0.837 | <0.001 |
| Body mass index | 0.479 | <0.001 | 0.505 | <0.001 |
| Albumin | 0.347 | 0.001 | 0.023 | 0.830 |
| Total lymphocyte count | 0.225 | 0.034 | 0.157 | 0.143 |
| Nutrition screening index score | 0.483 | <0.001 | 0.297 | 0.005 |
Nutritional, functional, and clinical variables in all patients (n=89) divided according to the median value of the phase angle (3.5×)
| PA ≤3.5× (n=44) | PA >3.5× (n=45) | P-value | |
|---|---|---|---|
| Age (y) | 70.3±3.7 | 60.2±13.7 | 0.001 |
| Sex (male/female) | 29/15 | 34/11 | 0.358 |
| APACHE II score | 22.7±10.5 | 17.5±7.8 | 0.010 |
| Nutritional variables | |||
| NSI score | 8.8±1.3 | 9.9±1.6 | <0.001 |
| Malnourished according to NSI score (%) | 19 (43.2) | 17 (37.8) | 0.669 |
| Phase angle (o) | 2.6±0.6 | 4.8±1.0 | <0.001 |
| Actual body weight (kg) | 55.0±9.8 | 63.0±11.5 | 0.001 |
| Body mass index (kg/m2) | 20.9±3.6 | 23.3±3.2 | 0.001 |
| Albumin (mg/dL) | 3.1±0.6 | 3.5±0.7 | 0.005 |
| Total lymphocyte count (mm3) | 1,076.0±743.9 | 1,400.6±1,023.1 | 0.090 |
| Fat-free mass (kg) | 44.8±8.2 | 51.6±10.3 | 0.001 |
| Clinical variables | |||
| ICU mortality (%) | 14 (31.8) | 9 (20.0) | 0.151 |
| Length of ICU stay (d) |
9.9±9.7 | 5.9±5.6 | 0.041 |
| Duration of mechanical ventilation (d) |
6.5±9.3 | 2.8±4.3 | 0.039 |
aICU survival subgroup: PA ≤3.5, n=30; PA >3.5, n=36.
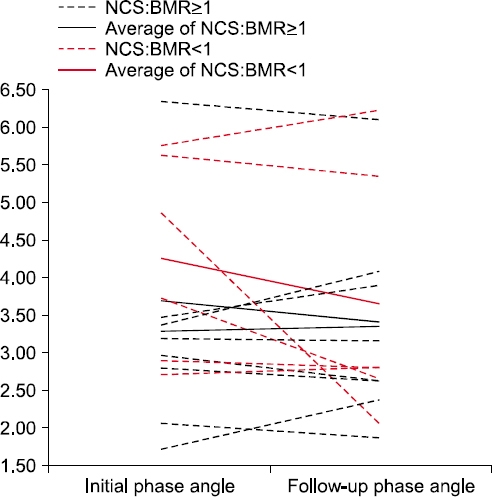
The changes in phase angle and nutritional markers divided according to the ratio of nutritional caloric support (NCS) to the basal metabolic rate (BMR) (ratio=1) in the repeated BIA group
| NCS:BMR ≥1 (n=9) | NCS:BMR <1 (n=6) | P-value | |||
|---|---|---|---|---|---|
| Initial | Post | Initial | Post | ||
| Age (y) | 69.2±12.6 | 60.0±15.6 | 0.228 | ||
| Sex (male/female) | 5/4 | 4/2 | 1.000 | ||
| APACHE II score | 25.0±10.5 | 21.5±8.4 | 0.506 | ||
| DM/liver disease | 3 | 1/2 | 0.758 | ||
| Basal metabolic rate | 1,316.3±165.3 | 1,427.8±227.5 | 0.290 | ||
| Nutritional markers | |||||
| Phase angle (°) | 3.3±1.3 | 3.4±1.3 | 4.2±1.3 | 3.7±1.7 | 0.139 |
| Malnourished according to NSI coding | 2 (22.2) | 5 (55.6) | 2 (33.3) | 3 (50.0) | 1.000 |
| Actual body weight (kg) | 55.1±12.6 | 54.7±12.1 | 61.6±11.9 | 60.8±11.2 | 0.669 |
| Body mass index (kg/m2) | 21.7±5.1 | 21.5±4.9 | 21.9±3.4 | 21.7±3.3 | 0.857 |
| Albumin (mg/dL) | 3.1±0.9 | 2.9±0.4 | 3.5±0.6 | 3.3±0.7 | 0.918 |
| Total lymphocyte count (mm3) | 1,675.3±1,100.9 | 1,488.2±882.8 | 1,265.9±1,252.5 | 1,032.9±806.7 | 0.945 |
| NSI score | 9.3±1.2 | 8.1±1.5 | 9.6±2.0 | 8.8±1.4 | 0.713 |
| Clinical variables | |||||
| ICU survival | 8 (88.9) | 4 (66.7) | 0.525 | ||
ICU survival subgroup, n=66.
ICU survival subgroup: PA ≤3.5, n=30; PA >3.5, n=36.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN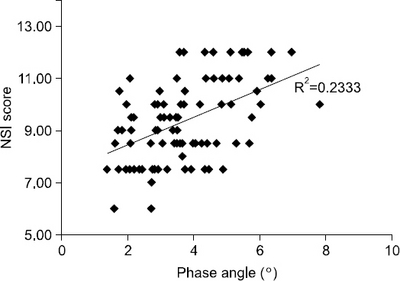
 Cite
Cite

