Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 8(2); 2017 > Article
- Review Article Enteral Nutrition in Liver Disease
- Kyung Sik Kim, M.D., Ph.D.
- 간질환 환자에서의 경장영양요법
- 김경 식, M.D., Ph.D.
-
Surgical Metabolism and Nutrition 2017;8(2):28-35.
DOI: https://doi.org/10.18858/smn.2017.8.2.28
Published online: December 30, 2017
Department of Surgery, Yonsei University College of Medicine, Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Severance Hospital, Seoul, Korea
- Correspondence to: Kyung Sik Kim, Department of Surgery, Yonsei University College of Medicine, Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Severance Hospital, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: kskim88@yuhs.ac
Copyright: © The Korean Society of Surgical Metabolism and Nutrition
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,293 Views
- 11 Download
Abstract
- Patients with chronic liver disease have a high risk to malnutrition. Proper nutrition should be provided through a proper nutritional assessment. Enteral nutrition is recommended as a nutritional supplement because it maintains the intestinal mucosa, reduces infectious complications, is less costly than parenteral nutrition, and is more physiological to use intestine. The purpose of this review is to define the nutritional deficiencies of patients with liver disease and to show the indications for enteral nutrition and to validate the efficacy of enteral nutrition. According to the various guidelines and researches, enteral nutrition is used as a solution to the nutritional problems caused by patients with liver disease. The optimal enteral formula will be selected on the nutritional problems. It is expected that the enteral nutrition will reduce especially postoperative complications, intraperitoneal complications, pneumonia, and wound infection. The enteral nutrition for patients with chronic liver disease should be actively implemented.
서론
본론
1) 유럽 경정맥 경장 영양학회 가이드라인(2006) [9]
2) 미국 경정맥 경장 영양학회 및 중환자 관리 의학 학회 가이드라인(2009) [10]
3) 미국 간학회와 위장관 미국 대학 협회 가이드라인(2010) [11]
4) 그 밖의 영양 지원 가이드라인
결론
- 1. Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev 2012;5:CD008344.Article
- 2. Kim ER. Enteral nutritional support in gastrointestinal and liver diseases. Korean J Gastroenterol 2015;65:354-60. ArticlePubMed
- 3. Harkness L. The history of enteral nutrition therapy: from raw eggs and nasal tubes to purified amino acids and early postoperative jejunal delivery. J Am Diet Assoc 2002;102:399-404. ArticlePubMed
- 4. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. ArticlePubMed
- 5. Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int 2012;11:586-93. ArticlePubMed
- 6. Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition 2013;29:1186-91. ArticlePubMed
- 7. Hasse JM, DiCecco SR. Enteral nutrition in chronic liver disease: translating evidence into practice. Nutr Clin Pract 2015;30:474-87. ArticlePubMed
- 8. Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract 2013;28:15-29. ArticlePubMedPDF
- 9. Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006;25:285-94. ArticlePubMed
- 10. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277-316. ArticlePubMed
- 11. O'Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Committee of the American Association for the Study of Liver Diseases;Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010;51:307-28. ArticlePubMed
- 12. Swain S, Krause T, Laramee P, Stewart S; Guideline Development Group. Diagnosis and clinical management of alcohol related physical complications: summary of NICE guidance. BMJ 2010;340:c2942.ArticlePubMed
- 13. SIGN. Mangement of hepatitis C. Edinburgh: Scottish Intercollegiate Guidelines Network; 2013. p. 133.Article
- 14. Koretz RL. The evidence for the use of nutritional support in liver disease. Curr Opin Gastroenterol 2014;30:208-14. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

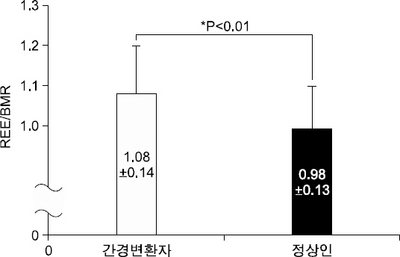
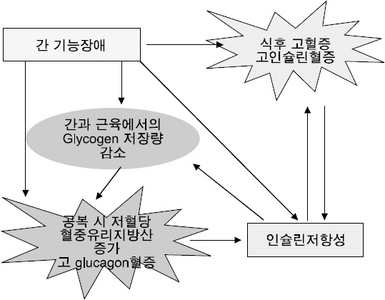
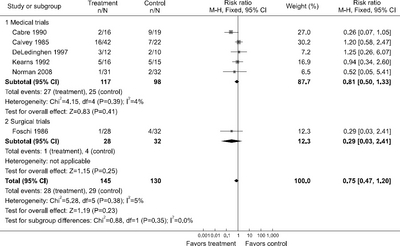
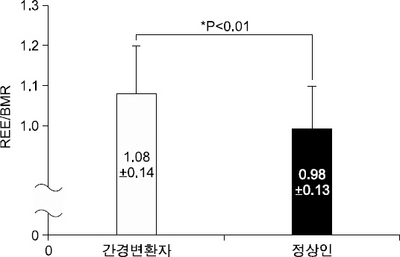
Fig. 1
Fig. 2
Fig. 3
Factors contributing to malnutrition in patients with chronic liver disease
| Factor | Mechanisms |
|---|---|
| Inadequate nutrient intake | ↑Levels of tumor necrosis factor-α and leptin → loss of appetite Ascites → impaired gastric expansion → early satiety, delayed gastric emptying, bloating, abdominal distention Hepatic encephalopathy → altered consciousness with decreased oral intake Alcohol intake replaces nutrition Nausea and vomiting Restrictive diets (low-sodium, low-protein, fluid restriction) Altered taste perception (zinc deficiency) Socioeconomic constraints |
| Metabolic alterations | Altered glucose, lipid, and protein metabolism Altered pattern of energy consumption Decreased glycogen levels and reduced ability to store nutrients Insulin resistance |
| Malabsorption | Bile salt deficiency in cholestatic liver disease and cholestasis Small bowel bacterial overgrowth Portal hypertensive enteropathy |
Tools for assessing oral protein-energy intake in end stage liver disease
| Assessment Tool | Method | Strengths | Limitations |
|---|---|---|---|
| 24-Hour recall | • Participant recalls all foods and beverages consumed over the previous 24 hours • Used to estimate protein-calorie intake |
• Low cost • Quick • No equipment required |
• May be inaccurate in those with poor memory or encephalopathy • Underreporting of portions and food items consumed may occur in women, those with body issues, or those who are overweight |
| Food frequency questionnaire | • Participant is given a list of foods/beverages and indicates how frequently these foods are consumed | • Low cost • Quick |
• May not represent foods typically consumed • High level of participant literacy required • Does not provide data on portion sizes or actual protein-energy intake |
| Calorie count | • A healthcare professional calculates protein-energy intake based on foods consumed | • Does not rely on patient’s recall • Low cost • No equipment required |
• Subjective • Portion sizes may not be standard or well documented • Often relies on nursing staff to complete |
| Food diary | • Patient or caregiver records foods eaten • Protein-energy intake is then calculated by a healthcare professional |
• Low cost • Does not require special equipment • Can be very accurate |
• Requires instruction by provider • Requires a higher level of literacy • Subjectivity may lead to inaccuracies • Typically underestimates energy intake • Time-consuming for provider to analyze intake |
Anthropometric assessment in end stage liver disease
| Tool | Method | Strengths | Limitations |
|---|---|---|---|
| Body mass index (BMI) | • Weight (kg)/height (m2) | • Easy to perform • No equipment required • Cost free |
• Inaccurate in patients with ascites or edema unless dry weight is available |
| Waist circumference | • Measures abdominal visceral adiposity | • Easily accessible • Low cost • Component involved in diagnosing metabolic syndrome |
• Not accurate in patients with ascites |
| Mid-arm circumference (MAC) | • Mid-arm is measured to assess muscle mass | • Low cost • Quick • Requires minimal equipment • Useful for assessing changes in muscle mass over time |
• Not a strong predictor of malnutrition |
| Skin fold | • Skin folds are measured using a caliper at various points of the body • Used to assess body fat |
• Low cost • Requires minimal equipment • Number of sites tested improves accuracy |
• Requires training for proper use • Conflicting reports of accuracy in predicting malnutrition in cirrhosis |
| Hand grip strength (HGS) | • A hand dynamometer is used to assess grip strength • Decreased grip strength is associated with malnutrition |
• Low cost • Requires a hand-grip dynamometer • Found to better predict complications of cirrhosis over the Subjective Global Assessment, BMI, skin fold, MAC, and BIA |
• Was not found to correlate with Child-Pugh score |
| Body cell mass (BCM) | • Validated marker used to assess body composition in the cirrhotic patient | • Very accurate even in the fluid-overloaded patient | • Expensive • Not readily available for clinical use and is typically used as a validation tool when analyzing other anthropometric assessments |
| Dual-energy X-ray absorptiometry (DEXA) | • Assesses body composition through a low-dose X-ray | • Gold-standard test | • Expensive • Not readily available |
| Bioelectrical impedance analysis (BIA) | • Measures body composition via an electrical current that estimates total body water, fat-free mass, and body fat | • Easily accessible • Correlates well with Child-Pugh score • Accurate in patients without ascites |
• Not accurate in patients with ascites |
| Air plethysmography | • Measures whole body density and subsequent calculation of body composition | • Noninvasive • Quick, convenient • Requires minimum compliance • Reliable • No water submersion |
• Varies among men and women • Limited availability |
Protein-energy requirements in end stage liver disease
| A.S.P.E.N./ESPEN | |
| Energy requirement based on dry weight or determined ideal body weight if ascites is present | 25∼40 kcal/kg/d |
| A.S.P.E.N. | |
| Stable and malnourished | REE×1.2∼1.4 |
| Without encephalopathy | REE×1.2∼1.4 1.0∼1.5 g/kg/d protein |
| Acute encephalopathy | REE×1.2∼1.4 0.6∼0.8 g/kg/d protein |
| ESPEN | |
| All stable cirrhosis patients | 35∼40 kcal/kg/d 1.0∼1.5 g/kg/d protein |
| Critically Ill | |
| ICU malnourished patients at risk for refeeding | 15∼20 kcal/kg/d 1.2 g/kg/d protein |
| ICU for maintenance caloric support | 25∼30 kcal/kg/d 1.5 g/kg/d protein |
| Catabolic | 35∼50 kcal/kg/d |
| Critically ill obese (body mass index <30) | Mifflin–St Jeor equation Indirect calorimetry for comorbidities 1.5∼2.0 g/kg/d protein ideal body weight |
Hepatic disease specific formulas
| Nutritional problems | Formula characteristics |
|---|---|
| Malnutrition | Calorically dense |
| Altered protein/carbohydrate metabolism | High branched-chain: aromatic amino acid ratio |
| Impaired urea synthesis | |
| Fat malabsorption | Fat system with MCT oil |
| Micronutrient deficiencies | Modified micronutrient profile |
| Fluid/sodium retention | Calorically dense, low sodium |
Enteral nutrition formula options for patients with chronic liver disease
| Enteral nutrition formula category | Indications and comments | Relative cost |
|---|---|---|
| Standard intact protein formulas | • Require normal digestion • Available in a variety of protein and calorie concentrations |
$ |
| Nutrient-dense formulas | • Require normal digestion • Generally available as 1.5∼2 kcal/ml concentrations • Useful in patients in whom fluid restriction is needed (eg, hypervolemic hyponatremia, fluid retention, reduced urine output, early satiety, high nutrition requirements) |
$ |
| Semi-elemental or partially hydrolyzed formulas | • Useful for patients who have impaired digestion • Available in a variety of protein and calorie concentrations • Often contain peptides and/or medium-chain triglycerides |
$$ |
| Elemental formulas | • Useful when digestion is impaired or a very-low-fat formula is preferred • Contain amino acids and dextrose (vs whole proteins and starches) • Usually high in carbohydrate, which could contribute to hyperglycemia in patients with insulin impairment • Usually hypertonic, which can reduce tolerance |
$$$ |
| Renal formulas | • Require normal digestion • Useful for patients with renal dysfunction and hyperkalemia or hyperphosphatemia • Usually fluid-restricted with reduced amounts of potassium and phosphorus |
$$ |
| Immune-enhancing formulas | • Require normal digestion • Have not been shown to be beneficial in patients with liver disease • Usually contain immune-enhancing nutrients such as fish oil, arginine, RNA • May affect insulin sensitivity and satiety • May temporarily increase serum ammonia levels but do not worsen symptoms of hepatic encephalopathy |
$$$ |
| BCAA formulas | • Controversial as to benefit, but American and European guidelines suggest consideration of BCAA formulas in patients with encephalopathy refractory to other treatments or with a protein intolerance • Contain higher proportion of BCAAs and reduced amounts of aromatic amino acids and methionine • Usually have reduced electrolyte content |
$$$ |
Results of meta-analyses of trials comparing enteral nutrition to no nutritional interventions in patients with various liver diseases
| Outcome | Cirrhosis | Alcoholic hepatitis | Liver transplantation | Obstructive jaundice |
|---|---|---|---|---|
| Mortality | 0.85 (0.54, 1.33); 4 (219) | 1.10 (0.61, 1.99); 2 (95) | No data | 0.29 (0.03, 2.41); 1 (60) |
| Appearance ascites | No data | No data | No data | No data |
| Resolution ascites | 0.86 (0.46, 1.62); 1 (29) | No data | No data | No data |
| Gastrointestinal bleeding | 1.17 (0.59, 2.34); 4 (215) | 2.88 (0.70, 11.87); 1 (64) | No data | No data |
| Appearance encephalopathy | 3.13 (0.64, 15.34); 2 (121) | 1.03 (0.26, 4.12); 2 (48) | 1.71 (0.46, 6.44); 1 (32) | No data |
| Resolution encephalopathy | No data | 1.57 (0.59, 4.13); 2 (47) | No data | No data |
| Infections | 0.91 (0.65, 1.29); 4 (211) | 0.96 (0.41, 2.25); 1/64 | 0.46 (0.15, 1.40); 1 (31) | 0.51 (0.18, 1.47); 1 (60) |
| Serum bilirubin | 0.37 (0.40, 1.15); 2 (162) | 5.90 (17.54, 5.74); 1 (31) | No data | No data |
| Duration of hospitalization | 1.08 (2.65, 4.80); 2/57 | No data | 9.80 (27.66, 8.06); 1 (31) | No data |
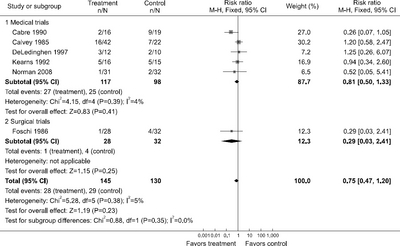
| Total postoperative complications | N/A | N/A | No data | 0.35 (0.16, 0.91); 1 (60) |
| Intra-abdominal postoperative complications | N/A | N/A | No data | 0.46 (0.10, 2.17); 1 (60) |
| Postoperative pneumonia | N/A | N/A | No data | 0.38 (0.02, 8.95); 1 (60) |
| Postoperative wound infections | N/A | N/A | No data | 0.46 (0.10, 2.17); 1 (60) |
Results of meta-analyses of trials comparing supplements to no nutritional interventions in patients with various liver diseases
| Outcomes | Cirrhosis | Hepatocellular carcinoma | Liver transplantation | Surgery | Hepatitis C treatment |
|---|---|---|---|---|---|
| Mortality | 0.53 (0.24, 1.15); 5 (205) | 1.18 (0.95,1.47); 4 (505) | 0.27 (0.06, 1.23); 1 (82) | 1.50 (0.37, 5.98); 3 (136) | 3.11 (0.13, 73.08); 1 (53) |
| Appearance ascites | 0.72 (0.36, 1.46); 2 (62) | 0.53 (0.30, 0.87); 2 (286) | No data | No data | No data |
| Resolution ascites | 4.16 (0.87, 19.84); 2 (29) | No data | No data | No data | No data |
| Gastrointestinal bleeding | 0.87 (0.45, 1.69); 3 (118) | 1.50 (0.53, 4.26); 2 (305) | No data | 1.10 (0.07, 16.43); 1 (44) | No data |
| Appearance encephalopathy | 0.87 (0.67, 1.14); 9 (332) | 0.75 (0.38, 1.48); 2 (305) | 0.43 (0.14, 1.32); 1 (29) | Not estimable (no events in 68 patients in 2 trials) | No data |
| Resolution encephalopathy | 3.75 (1.15, 12.18) 2 (53) | No data | No data | No data | No data |
| Infections | 0.50 (0.24, 1.03); 3 (184) | 0.35 (0.01, 8.34); 1 (84) | No data | 0.86 (0.44, 1.67); 3 (94) | No data |
| Serum bilirubin | 0.24 (−2.00, 2.51); 2 (87) | No data | No data | No data | No data |
| Duration of hospitalization | −8.00 (−17.54, 1.54); 1 (36) | See text | No data | See text | No data |
| Total postoperative complications | N/A | N/A | No data | 0.85 (0.66, 1.10); 4 (162) | N/A |
| Intra-abdominal postoperative complications | N/A | N/A | No data | 0.30 (0.05, 1.74); 2 (68) | N/A |
| Postoperative pneumonia | N/A | N/A | No data | 0.55 (0.26, 2.29); 2 (68) | N/A |
| Postoperative wound infections | N/A | N/A | No data | 0.77 (0.26, 2.29); 2 (68) | N/A |

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN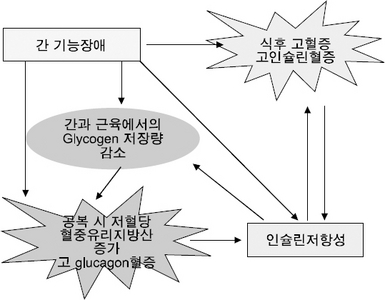
 Cite
Cite

