Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Surgical Metabolism and Nutrition > Volume 10(2); 2019 > Article
- ORIGINAL ARTICLE Clinical Implications of the Cut-off Value of the Preoperative Prognostic Nutritional Index in Patients with Early Stage Gastric Cancer
- Ji Hye Jung, M.D.1, Ji Yeong An, M.D., Ph.D.1, You Na Kim, M.D.2, Min Gew Choi, M.D., Ph.D.1, Jun Ho Lee, M.D., Ph.D.1, Tae Sung Sohn, M.D., Ph.D.1, Jae Moon Bae, M.D., Ph.D.1, Sung Kim, M.D., Ph.D.1
-
Surgical Metabolism and Nutrition 2019;10(2):59-65.
DOI: https://doi.org/10.18858/smn.2019.10.2.59
Published online: December 30, 2019
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine
Department of Surgery, Korea University College of Medicine, Seoul, Korea
Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine
Department of Surgery, Korea University College of Medicine, Seoul, Korea
- Correspondence to: Ji Yeong An, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-0884, Fax: +82-2-3410-6981, E-mail: jar319.an@samsung.com
Copyright: © The Korean Society of Surgical Metabolism and Nutrition
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,117 Views
- 1 Download
Abstract
-
Purpose: The perioperative nutritional status is a potential prognostic factor in gastric cancer patients. This study assessed the optimal cut-off value of the prognostic nutritional index (PNI) for predicting the survival of patients with early stage gastric cancer and evaluated its power for predicting the survival after gastric cancer surgery.
-
Materials and Methods: This study reviewed the data of 8,014 patients with stage T1N0~1M0 and T2~3N0M0 gastric cancer who underwent a curative gastrectomy without adjuvant chemotherapy between January 2006 and December 2015. The log-rank test on SAS was conducted to determine the preoperative PNI cut-off value that indicated the most significant difference in survival, and the clinical features and oncological outcomes were analyzed according to the cut-off value of the preoperative PNI.
-
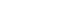
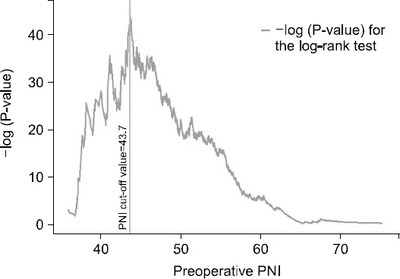
Results: The preoperative PNI cut-off value that indicated the most significant difference in survival was 43.7. Using this cut-off value, patients were classified into high PNI and low PNI groups. The five-year overall survival rate was 96.9% and 81.5% for the high and low PNI group, respectively (P<0.001). Considering each stage (Ia, Ib, and IIa), the overall survival rates were significantly higher for the high PNI group than the low PNI group. Multivariable analysis revealed the cut-off value of the preoperative PNI to be among the independent risk factors for survival.
-
Conclusions: The cut-off value of the preoperative PNI that could be used to determine the significant differences in the survival of patients with early stage gastric cancer was identified and proven to have a significant impact on predicting survival.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
DISCLOSURE
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. ArticlePubMedPDF
- 2. Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology 2017;152:1319-28. e7.ArticlePubMed
- 3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3):analysis of individual records for 37 513?025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. ArticlePubMedPMC
- 4. Rogers C. Postgastrectomy nutrition. Nutr Clin Pract 2011;26:126-36. ArticlePubMedPDF
- 5. Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr 2019;38:870-6. ArticlePubMed
- 6. Li J, Xu R, Hu DM, Zhang Y, Gong TP, Wu XL. Prognostic nutritional index predicts outcomes of patients after gastrectomy for cancer:a systematic review and meta-analysis of nonrandomized studies. Nutr Cancer 2019;71:557-68. ArticlePubMed
- 7. Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer:analysis of a multi-institution dataset. Dig Surg doi:10.1159/000497454. [In press]. ArticlePDF
- 8. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol 2019;2013;20:2647-54. ArticlePubMedPDF
- 9. Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. New York: Wiley-Blackwelly; 2011. Article
- 10. Loan BTH, Nakahara S, Tho BA, Dang TN, Anh LN, Huy ND, et al. Nutritional status and postoperative outcomes in patients with gastrointestinal cancer in Vietnam:a retrospective cohort study. Nutrition 2018;48:117-21. ArticlePubMed
- 11. Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine (Baltimore) 2016;95:e3781.ArticlePubMedPMC
- 12. Lee H, Cho YS, Jung S, Kim H. Effect of nutritional risk at admission on the length of hospital stay and mortality in gastrointestinal cancer patients. Clin Nutr Res 2013;2:12-8. ArticlePubMedPMC
- 13. van Stijn MF, Korkic-Halilovic I, Bakker MS, van der Ploeg T, van Leeuwen PA, Houdijk AP. Preoperative nutrition status and postoperative outcome in elderly general surgery patients:a systematic review. JPEN J Parenter Enteral Nutr 2013;37:37-43. ArticlePubMed
- 14. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol 2015;22(Suppl 3):S778-85. ArticlePubMedPDF
- 15. Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy:a systematic review and meta-analysis. BMC Gastroenterol 2018;18:11.ArticlePubMedPMCPDF
- 16. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels:a propensity score matching analysis. BMC Cancer 2018;18:285.ArticlePubMedPMCPDF
- 17. Lee JY, Kim HI, Kim YN, Hong JH, Alshomimi S, An JY, et al. Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy:a retrospective analysis of 7781 gastric cancer patients. Medicine (Baltimore) 2016;95:e3539.ArticlePubMedPMC
- 18. Song S, Liu H, Xue Y. [Clinical significance of prognostic nutritional index in patients with advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:180-4; Chinese. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

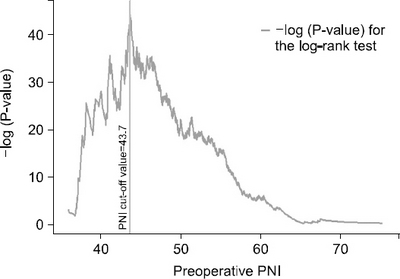
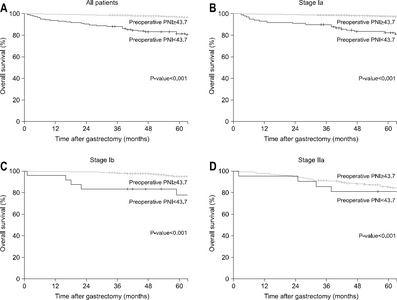
Fig. 1
Fig. 2
Baseline clinicopathological characteristics of the entire patient population (n=8014)
| Mean±SD | |
|---|---|
| Age (years) | 57.2±11.6 |
| Sex | |
| Male | 5118 (63.9) |
| Female | 2896 (36.1) |
| Preoperative body weight (kg) | 64.4±10.6 |
| BMI (kg/m2) | 24.1±3.0 |
| ASA score | |
| 1 | 3713 (46.3) |
| 2 | 4125 (51.5) |
| 3 | 176 (2.2) |
| Preoperative albumin level (g/dL) | 4.4±0.4 |
| Preoperative hemoglobin level (g/dL) | 13.9±1.7 |
| Preoperative total protein level (g/dL) | 7.0±0.5 |
| Preoperative cholesterol level (mg/dL) | 185.4±36.1 |
| Preoperative PNI | 54.9±5.1 |
| Resection extent | |
| Subtotal gastrectomy | 6668 (83.2) |
| Total gastrectomy | 1346 (16.8) |
| Surgical approach | |
| Open | 6208 (77.5) |
| Minimally invasive | 1806 (22.5) |
| Tumor location in the stomach | |
| Lower | 4594 (57.3) |
| Middle | 2454 (30.6) |
| Upper | 960 (12.0) |
| Whole | 6 (0.1) |
| Tumor size (cm) | 3.0±1.9 |
| T stage | |
| T1 | 7126 (88.9) |
| T2 | 698 (8.7) |
| T3 | 190 (2.4) |
| N stage | |
| N0 | 7707 (96.2) |
| N1 | 269 (3.3) |
| N2 | 38 (0.5) |
| WHO classification | |
| Differentiated | 3449 (43.0) |
| Undifferentiated | 4565 (57.0) |
| Lauren classification | |
| Intestinal | 3676 (45.9) |
| Diffuse | 3434 (42.8) |
| Mixed | 904 (11.3) |
BMI = body mass index; ASA = American Society of Anesthesiologists; PNI = prognostic nutritional index; WHO = World Health Organization.
Characteristics of the two groups
| Pre-op PNI<43.7 (n=144) | Pre-op PNI≥43.7 (n=7870) | P-value | |
|---|---|---|---|
| Age (years) | 65.9±11.1 | 57.0±11.6 | <0.001 |
| Sex | 0.856 | ||
| Male | 93 (64.6) | 5025 (63.9) | |
| Female | 51 (35.4) | 2845 (36.1) | |
| Preoperative body weight (kg) | 59.9±9.9 | 64.5±10.6 | <0.001 |
| BMI (kg/m2) | 23.3±3.2 | 24.1±3.0 | <0.001 |
| ASA score | <0.001 | ||
| 1 | 29 (20.1) | 3684 (46.8) | |
| 2 | 90 (62.5) | 4035 (51.3) | |
| 3 | 25 (17.4) | 151 (1.9) | |
| Preoperative albumin level (g/dL) | 3.4±0.3 | 4.4±0.3 | <0.001 |
| Preoperative hemoglobin level (g/dL) | 11.5±2.0 | 14.0±1.6 | <0.001 |
| Preoperative total protein level (g/dL) | 6.1±0.7 | 7.0±0.5 | <0.001 |
| Preoperative cholesterol level (mg/dL) | 152.0±34.8 | 186.0±35.9 | <0.001 |
| Preoperative PNI | 40.7±2.8 | 55.1±4.8 | NA |
| Resection extent | 0.683 | ||
| Subtotal gastrectomy | 118 (81.9) | 6550 (83.2) | |
| Total gastrectomy | 26 (18.1) | 1320 (16.8) | |
| Surgical approach | <0.001 | ||
| Open | 139 (96.5) | 6069 (77.1) | |
| Minimally invasive | 5 (3.5) | 1801 (22.9) | |
| Tumor location in the stomach | 0.830 | ||
| Lower | 86 (59.7) | 4508 (57.3) | |
| Middle | 44 (30.6) | 2410 (30.6) | |
| Upper | 14 (9.7) | 946 (12.0) | |
| Whole | 0 (0.0) | 6 (0.1) | |
| Tumor size (cm) | 4.2±2.8 | 3.0±1.9 | <0.001 |
| T stage | <0.001 | ||
| T1 | 103 (71.5) | 7023 (89.2) | |
| T2 | 24 (16.7) | 674 (8.6) | |
| T3 | 17 (11.8) | 173 (2.2) | |
| N stage | 0.797 | ||
| N0 | 137 (95.1) | 7570 (96.2) | |
| N1 | 6 (4.2) | 263 (3.3) | |
| N2 | 1 (0.7) | 37 (0.5) | |
| WHO classification | <0.001 | ||
| Differentiated | 98 (68.1) | 3351 (42.6) | |
| Undifferentiated | 46 (31.9) | 4519 (57.4) | |
| Lauren classification | <0.001 | ||
| Intestinal | 99 (68.8) | 3577 (45.5) | |
| Diffuse | 33 (22.9) | 3401 (43.2) | |
| Mixed | 12 (8.3) | 892 (11.3) |
Pre-op = preoperative; PNI = prognostic nutritional index; BMI = body mass index; ASA = American Society of Anesthesiologists; WHO = World Health Organization.
Univariable and multivariable analyses for overall survival
| Risk factor | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Age | 1.100 | 1.088 | 1.112 | <0.001 | 1.084 | 1.071 | 1.097 | <0.001 |
| Sex | ||||||||
| Male | ref. | ref. | ||||||
| Female | 0.411 | 0.318 | 0.531 | <0.001 | 0.428 | 0.331 | 0.555 | <0.001 |
| Preoperative PNI | ||||||||
| <43.7 | 7.109 | 5.175 | 9.766 | <0.001 | 2.925 | 2.103 | 4.067 | <0.001 |
| ≥43.7 | ref. | ref. | ||||||
| BMI | 0.924 | 0.892 | 0.957 | <0.001 | 0.940 | 0.906 | 0.976 | 0.001 |
| Resection extent | ||||||||
| Subtotal | ref. | ref. | ||||||
| Total | 1.338 | 1.044 | 1.714 | 0.021 | 1.134 | 0.882 | 1.457 | 0.327 |
| ASA score | ||||||||
| 1 | ref. | ref. | ||||||
| 2 | 2.541 | 2.003 | 3.222 | <0.001 | 1.160 | 0.899 | 1.497 | 0.254 |
| 3 | 10.151 | 7.011 | 14.698 | <0.001 | 2.572 | 1.738 | 3.807 | <0.001 |
| Stage | ||||||||
| Ia | ref. | ref. | ||||||
| Ib | 2.044 | 1.550 | 2.696 | <0.001 | 1.404 | 1.061 | 1.857 | 0.018 |
| IIa | 5.050 | 3.792 | 6.725 | <0.001 | 2.455 | 1.826 | 3.302 | <0.001 |
| WHO classification | ||||||||
| Differentiated | ref. | ref. | ||||||
| Undifferentiated | 0.477 | 0.388 | 0.587 | <0.001 | 1.200 | 0.869 | 1.657 | 0.267 |
| Lauren classification | ||||||||
| Intestinal | ref. | ref. | ||||||
| Diffuse | 0.363 | 0.285 | 0.463 | <0.001 | 0.690 | 0.477 | 0.998 | 0.049 |
| Mixed | 0.617 | 0.432 | 0.883 | 0.008 | 0.765 | 0.506 | 1.157 | 0.205 |
| Postoperative PNI | ||||||||
| 3 months | 0.887 | 0.869 | 0.906 | <0.001 | ||||
| 6 months | 0.883 | 0.861 | 0.907 | <0.001 | ||||
| 12 months | 0.897 | 0.872 | 0.923 | <0.001 | ||||
HR = hazard ratio; CI = confidence interval; PNI = prognostic nutritional index; BMI = body mass index; ASA = American Society of Anesthesiologists; WHO = World Health Organization.
BMI = body mass index; ASA = American Society of Anesthesiologists; PNI = prognostic nutritional index; WHO = World Health Organization.
Pre-op = preoperative; PNI = prognostic nutritional index; BMI = body mass index; ASA = American Society of Anesthesiologists; WHO = World Health Organization.
HR = hazard ratio; CI = confidence interval; PNI = prognostic nutritional index; BMI = body mass index; ASA = American Society of Anesthesiologists; WHO = World Health Organization.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite