Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 14(1); 2022 > Article
- Review Article Effect of Probiotics/Synbiotics on Postoperative Outcomes in Patients Undergoing Abdominal Surgery
-
In Ja Park, M.D., Ph.D.

-
DOI: https://doi.org/10.15747/ACNM.2022.14.1.10
Published online: June 1, 2022
Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding author: In Ja Park E-mail ipark@amc.seoul.kr ORCID https://orcid.org/0000-0001-5355-3969
© The Korean Society of Surgical Metabolism and Nutrition and The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 12,180 Views
- 127 Download
- 3 Crossref
- Abstract
- INTRODUCTION
- ALTERATIONS IN GUT MICROBIOTA AFTER ABDOMINAL SURGERY
- INFLUENCE OF PROBIOTICS/SYNBIOTICS ON GUT MICROBIOTA COMPOSITION IN PATIENTS WHO RECEIVED ABDOMINAL SURGERY
- ASSOCIATION BETWEEN POSTOPERATIVE COMPLICATIONS AND MICROBIOTA IN PATIENTS WHO RECEIVED ABDOMINAL SURGERY
- EFFECT OF PROBIOTICS/SYNBIOTICS ON POSTOPERATIVE COMPLICATIONS IN SURGICAL PATIENTS
- CONCLUSION
- Notes
- References
Abstract
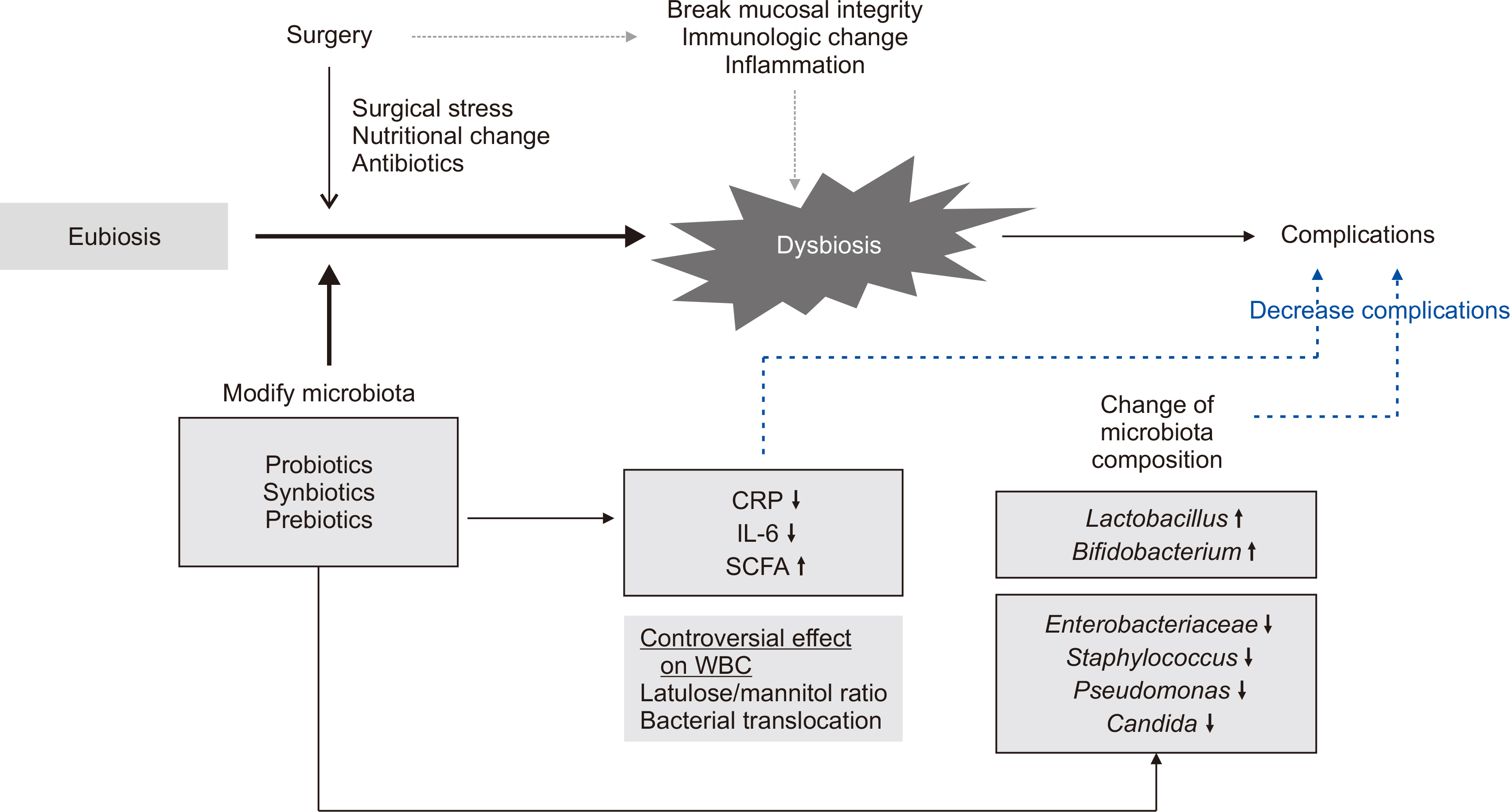
- Environmental factors, drugs, diet, and surgery alter the composition of the gut microbiota leading to the production of different metabolites or toxins that can cause disease or delay postoperative recovery. Surgical damage leads to gut barrier disruption, increased intestinal permeability, gut microbial imbalance, and immunologic compromise of the host with subsequent bacterial translocation from the gastrointestinal tract to systemic circulation. Therefore, perioperative stabilization of the intestinal microbiota is a potential method of reducing postoperative complication rates. Probiotics have been proposed as a viable option for prophylaxis of postoperative infections through increased intestinal motility to prevent bacterial overgrowth, improve gut barrier function, and modulate immune response. This review investigates microbial changes after surgery and the influence of probiotics on postoperative microbial composition. Infectious postoperative complications and immunologic changes related to probiotics/synbiotics were also reviewed in patients who underwent abdominal surgery.
INTRODUCTION
ALTERATIONS IN GUT MICROBIOTA AFTER ABDOMINAL SURGERY
INFLUENCE OF PROBIOTICS/SYNBIOTICS ON GUT MICROBIOTA COMPOSITION IN PATIENTS WHO RECEIVED ABDOMINAL SURGERY
ASSOCIATION BETWEEN POSTOPERATIVE COMPLICATIONS AND MICROBIOTA IN PATIENTS WHO RECEIVED ABDOMINAL SURGERY
EFFECT OF PROBIOTICS/SYNBIOTICS ON POSTOPERATIVE COMPLICATIONS IN SURGICAL PATIENTS
CONCLUSION
CONFLICTS OF INTEREST
The author of this manuscript has no conflicts of interest to disclose.
FUNDING
None.
| Author | Year | Patient number | Indication for surgery | Duration of treatment (day) | Included strains (substrates in synbiotics) |
|---|---|---|---|---|---|
| Synbiotics | |||||
| Rayes et al. [31] | 2002 | 90 | Major surgery | 5a | Lactobacillus plantarum 299v (oat fiber) |
| Kanazawa et al. [16] | 2005 | 55 | Biliary cancer | 14a | Bifidobacterium breve, Lactobacillus casei (galacto-oligosaccharides) |
| Sugawara et al. [17] | 2006 | 101 | Liver/biliary surgery | 28b (14/14) | Lactobacillus casei Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Rayes et al. [27] | 2007 | 89 | Pancreatic surgery | 9b (1/8) | Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus plantarum, Lactobacillus paracasei (beta glucan, inulin, pectin, resistant starch) |
| Horvat et al. [24] | 2010 | 76 | Colorectal surgery | 3a | Mixture of 4 Lactobacilli (beta-glucan, inulin, starch, pectin) |
| Eguchi et al. [36] | 2011 | 50 | Liver transplantation | Bifidobacterium breve, Lactobacillus casei (galacto-oligosaccharides) | |
| Usami et al. [12] | 2011 | 67 | Liver resection | 26b (14/12) | Yakult BL seichoyaku (galacto-oligosaccharides) |
| Tanaka et al. [13] | 2012 | 64 | Esophagectomy | 22b (1/21) | Lactobacillus casei Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Okazaki et al. [14] | 2013 | 53 | Upper GI, hepatobiliary, pancreatic cancer | 17b (7/10) | Lactobacillus casei Shirota, Bifidobacterium breve Yakult (galacto-oligosaccharides) |
| Sommacal et al. [32] | 2015 | 54 | Periampullary tumor | 14b (4/10) | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium bifidum (fructo-oligosaccharides) |
| Komatsu et al. [33] | 2016 | 379 | Colorectal surgery (laparoscopic) | 9~18b (7~11/2~7) | Lactobacillus casei Strain Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Yokoyama et al. [28] | 2017 | 50 | Pancreatico-duodenectomy | 21b (7/14) | Lactobacillus casei Strain Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Flesch et al. [35] | 2017 | Colorectal cancer | 19b (5/14) | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium lactis (fructo-oligosaccharides) | |
| Polakowski et al. [34] | 2019 | 77 | Colorectal cancer | 7a | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium lactis (fructo-oligosaccharide) |
| Park et al. [40] | 2020 | 68 | Colorectal cancer | 28b (7/21) | Bifidobacterium animalis subsp. lactis HY8002, Lactobacillus casei HY2782, Lactobacillus plantarum HY7712 (lactose, xylitol, maltitol) |
| Probiotics | |||||
| McNaught et al. [25] | 2002 | 129 | Major surgery | 14b (9/5) | Lactobacillus plantarum 299v |
| Nomura et al. [37] | 2007 | 70 | Pancreatic surgery | Variableb | Enterococcus faecalis, Clostridium butyricum, Bacillus mesentericus |
| Liu et al. [18] | 2011 | 120 | Colorectal surgery | 16b (6/10) | Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum |
| Zhang et al. [15] | 2012 | 60 | Colorectal cancer | 3a | Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis |
| Liu et al. [26] | 2013 | 161 | Colorectal cancer | 16b (6/10) | Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum |
| Sadahiro et al. [29] | 2014 | 310 | Colorectal cancer | 7~23b (2~8/5~15) | Bifidobacteria |
| Kotzampassi et al. [38] | 2015 | 164 | Colorectal cancer | 14a | Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, Saccharomyces boulardii |
| Yang et al. [30] | 2016 | 92 | Colorectal cancer | 12b (5/7) | Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis |
| Grąt et al. [39] | 2017 | 55 | Liver transplantation | Variablea | Lactobacillus lactis, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium bifidum |
| Author | Probiotics/synbiotics | Control | |||
|---|---|---|---|---|---|
|
|
|
||||
| Increase | Decrease | Increase | Decrease | ||
| Synbiotics | |||||
| Kanazawa et al. [16] | Lactobacillus, Bifidobacteriuma Enterococci | Enterobacteriaceae, Pseudomonas, Candidaa, Enterococci | |||
| Sugawara et al. [17] | Lactobacillus, Bifidobacteriuma | Candida | |||
| Eguchi et al. [36] | No significant difference between groups | ||||
| Usami et al. [12] | Candida | Bacteroidaceae, Bifidobacterium | |||
| Tanaka et al. [13] | Bifidobacterium, Lactobacillusb | Enterobacteriaceaea | |||
| Okazaki et al. [14] | Bifidobacteriuma | Enterobacteriaceae, Staphylococcusa | Bifidobacterium | ||
| Komatsu et al. [33] | Clostridium leptum subgroup, Bifidobacterium, Lactobacillusa | Enterobacteriaceae, Staphylococcus, Pseudomonasa | Enterobacteriaceae, Staphylococcus, Pseudomonas, Clostridium difficilea | Clostridium coccoides group, Clostridium leptum subgroup, Bacteroides fragilis group, Bifidobacterium, Prevotella, Lactobacillusa | |
| Yokoyama et al. [28] | Bifidobacterium, Lactobacillusa | Enterobacteriaceae, Pseudomonasa | Pseudomonas, Staphylococcus, Enterobacteriaceaea | ||
| Probiotics | |||||
| Liu et al. [18] | Bifidobacteriuma, Enterococci | Enterobacteriaceae, Pseudomonas, Candidaa | Enterobacteriaceae, Pseudomonas, Candidaa, Enterococci | ||
| Zhang et al. [15] | Bifidobactrium longumb | Escherichia colib | Bifidobacterium–Escherichia coli ratio inversionb | ||
| Grąt et al. [39] | Enterococcusa, Lactobacillus | ||||
| Author | Infectious complications (%) | Mortality (%) | |||
|---|---|---|---|---|---|
|
|
|
||||
| Probiotics/synbiotics | Control | Probiotics/synbiotics | Control | ||
| Kanazawa et al. [16] | 19.0 | 52.2 | 0 | 0 | |
| Rayes et al. [27] | 12.5 | 40.0 | 2.5 | 2.5 | |
| Horvat et al. [24] | 0 | 5.0 | 0 | 0 | |
| Eguchi et al. [36] | 4.0 | 24.0 | 12.0 | 12.0 | |
| Usami et al. [12] | 0 | 17.2 | 0 | 0 | |
| Okazaki et al. [14] | 24 .0 | 47.8 | 0 | 0 | |
| Sommacal et al. [32] | 26.1 | 69.6 | 0 | 10.0 | |
| Komatsu et al. [33] | 17.2 | 22.7 | 0 | 0 | |
| Yokoyama et al.[28] | 40.9 | 36.4 | 0 | 4.5 | |
| Flesch et al. [35] | 2.0 | 21.4 | - | - | |
| Polakowski et al. [34] | 2.8 | 18.9 | 0 | 8.1 | |
| Park et al. [40] | 6.0 | 28.5 | - | - | |
| McNaught et al. [25] | 10.9 | 15.4 | 10.9 | 3.1 | |
| Nomura et al. [37] | 23.3 | 52.9 | 0 | 2.9 | |
| Liu et al. [18] | 14.0 | 46.0 | 0 | 0 | |
| Zhang et al. [15] | 10.0 | 33.3 | - | - | |
| Liu et al. [26] | 54.7 | 73.3 | 0 | 0 | |
| Sadahiro et al. [29] | 24.0 | 25.3 | - | - | |
| Kotzampassi et al. [38] | 19.0 | 28.8 | - | 14.0 | |
| Yang et al. [30] | 10.0 | 30.0 | - | - | |
| Grąt et al. [39] | 25.0 | 19.2 | - | - | |
- 1. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012;13:260-70. ArticlePubMedPMCPDF
- 2. Morowitz MJ, Babrowski T, Carlisle EM, Olivas A, Romanowski KS, Seal JB, et al. The human microbiome and surgical disease. Ann Surg 2011;253:1094-101. ArticlePubMedPMC
- 3. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369-79. ArticlePubMed
- 4. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 2019;4:293-305. ArticlePubMedPMCPDF
- 5. Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun 2020;11:855.ArticlePubMedPMCPDF
- 6. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73.ArticlePubMedPMCPDF
- 7. Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 2019;11:2862.ArticlePubMedPMC
- 8. Codella R, Luzi L, Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis 2018;50:331-41. ArticlePubMed
- 9. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis 2016;34:260-8. ArticlePubMedPDF
- 10. Branch-Elliman W, O'Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019;154:590-8. ArticlePubMedPMC
- 11. Ohigashi S, Sudo K, Kobayashi D, Takahashi T, Nomoto K, Onodera H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg 2013;17:1657-64. ArticlePubMedPDF
- 12. Usami M, Miyoshi M, Kanbara Y, Aoyama M, Sakaki H, Shuno K, et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J Parenter Enteral Nutr 2011;35:317-28. ArticlePubMedPDF
- 13. Tanaka K, Yano M, Motoori M, Kishi K, Miyashiro I, Ohue M, et al. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: a prospective randomized controlled trial. Surgery 2012;152:832-42. ArticlePubMed
- 14. Okazaki M, Matsukuma S, Suto R, Miyazaki K, Hidaka M, Matsuo M, et al. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: a prospective, randomized control trial. Nutrition 2013;29:1224-30. ArticlePubMed
- 15. Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 2012;343:199-205. ArticlePubMed
- 16. Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 2005;390:104-13. ArticlePubMedPDF
- 17. Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 2006;244:706-14. ArticlePubMedPMC
- 18. Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Aliment Pharmacol Ther 2011;33:50-63. ArticlePubMed
- 19. Mitsuoka T. Significance of dietary modulation of intestinal flora and intestinal environment. Biosci Microflora 2000;19:15-25. Article
- 20. Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010;7:503-14. ArticlePubMedPMCPDF
- 21. Food and Agriculture Organization of the United Nations (FAO). World Health Organization (WHO). c2001. Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria; 2001 Oct 1-4; Córdoba, Argentina. FAO, WHO; Rome, Geneva: p. 32.Article
- 22. Food and Agriculture Organization of the United Nations (FAO). World Health Organization (WHO). c2002. Guidelines for the evaluation of probiotics in food; 2002 Apr 30-May 1; London (ON), Canada. FAO, WHO; Rome, Geneva: p. 10.Article
- 23. Natural Health Products Directorate. 2006. Evidence for safety and efficacy of finished natural health products [Internet]. Natural Health Products Directorate Health; Ottawa: Available from: https://publications.gc.ca/collections/collection_2007/hc-sc/H164-39-2006E.pdf. [cited 2021 Dec 24 Article
- 24. Horvat M, Krebs B, Potrc S, Ivanecz A, Kompan L. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien Klin Wochenschr 2010;122 Suppl 2:26-30. ArticlePubMedPDF
- 25. McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 2002;51:827-31. ArticlePubMedPMC
- 26. Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 2013;97:117-26. ArticlePubMed
- 27. Rayes N, Seehofer D, Theruvath T, Mogl M, Langrehr JM, Nüssler NC, et al. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg 2007;246:36-41. ArticlePubMedPMC
- 28. Yokoyama Y, Asahara T, Nomoto K, Nagino M. Effects of synbiotics to prevent postoperative infectious complications in highly invasive abdominal surgery. Ann Nutr Metab 2017;71 Suppl 1:23-30. ArticlePubMedPDF
- 29. Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki T, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery 2014;155:493-503. ArticlePubMed
- 30. Yang Y, Xia Y, Chen H, Hong L, Feng J, Yang J, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget 2016;7:8432-40. ArticlePubMedPMC
- 31. Rayes N, Hansen S, Seehofer D, Müller AR, Serke S, Bengmark S, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition 2002;18:609-15. ArticlePubMed
- 32. Sommacal HM, Bersch VP, Vitola SP, Osvaldt AB. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: a randomized, double-blind clinical trial. Nutr Cancer 2015;67:457-62. ArticlePubMed
- 33. Komatsu S, Sakamoto E, Norimizu S, Shingu Y, Asahara T, Nomoto K, et al. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: a randomized controlled trial. Surg Today 2016;46:479-90. ArticlePubMedPDF
- 34. Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Ligocki Campos AC. Impact of the preoperative use of synbiotics in colorectal cancer patients: a prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019;58:40-6. ArticlePubMed
- 35. Flesch AT, Tonial ST, Contu PC, Damin DC. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev Col Bras Cir 2017;44:567-73. ArticlePubMed
- 36. Eguchi S, Takatsuki M, Hidaka M, Soyama A, Ichikawa T, Kanematsu T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. Am J Surg 2011;201:498-502. ArticlePubMed
- 37. Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, et al. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 2007;54:661-3.ArticlePubMed
- 38. Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg 2015;39:2776-83. ArticlePubMedPDF
- 39. Grąt M, Wronka KM, Lewandowski Z, Grąt K, Krasnodębski M, Stypułkowski J, et al. Effects of continuous use of probiotics before liver transplantation: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2017;36:1530-9. ArticlePubMed
- 40. Park IJ, Lee JH, Kye BH, Oh HK, Cho YB, Kim YT, et al. Effects of probiotics on the symptoms and surgical outcomes after anterior resection of colon cancer (POSTCARE): a randomized, double-blind, placebo-controlled trial. J Clin Med 2020;9:2181.ArticlePubMedPMC
- 41. Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 2004;173:4137-46. ArticlePubMedPDF
- 42. Bachmann R, Leonard D, Delzenne N, Kartheuser A, Cani PD. Novel insight into the role of microbiota in colorectal surgery. Gut 2017;66:738-49. ArticlePubMed
- 43. Reinhardt C, Bergentall M, Greiner TU, Schaffner F, Ostergren-Lundén G, Petersen LC, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012;483:627-31. ArticlePubMedPMCPDF
- 44. Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, et al. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol 2005;288:G696-704. ArticlePubMed
- 45. Snijders HS, Wouters MW, van Leersum NJ, Kolfschoten NE, Henneman D, de Vries AC, et al. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol 2012;38:1013-9. ArticlePubMed
- 46. Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. Sepsis in general surgery: the 2005-2007 national surgical quality improvement program perspective. Arch Surg 2010;145:695-700. ArticlePubMed
- 47. Vogel TR, Dombrovskiy VY, Carson JL, Graham AM, Lowry SF. Postoperative sepsis in the United States. Ann Surg 2010;252:1065-71. ArticlePubMedPMC
- 48. Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol 2017;30:45-53. ArticlePubMedPMC
- 49. Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The "undrained abscess" of multiple organ failure. Ann Surg 1993;218:111-9. ArticlePubMedPMC
- 50. Gianotti L, Morelli L, Galbiati F, Rocchetti S, Coppola S, Beneduce A, et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol 2010;16:167-75. ArticlePubMedPMC
- 51. Defazio J, Fleming ID, Shakhsheer B, Zaborina O, Alverdy JC. The opposing forces of the intestinal microbiome and the emerging pathobiome. Surg Clin North Am 2014;94:1151-61. ArticlePubMedPMC
- 52. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol 2017;14:43-54. ArticlePubMedPDF
- 53. Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014;2:35.ArticlePubMedPMCPDF
- 54. Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One 2015;10:e0129996. ArticlePubMedPMC
- 55. Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. 2018;The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10:988.ArticlePubMedPMC
- 56. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011;469:543-7. ArticlePubMedPDF
- 57. Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011;17:1519-28. ArticlePubMedPMC
- 58. Seal JB, Morowitz M, Zaborina O, An G, Alverdy JC. The molecular Koch's postulates and surgical infection: a view forward. Surgery 2010;147:757-65. ArticlePubMed
- 59. Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch M, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One 2012;7:e44326. ArticlePubMedPMC
- 60. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. 2013;Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446-50. ArticlePubMed
- 61. Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010;1801:1175-83. ArticlePubMed
- 62. Lawhon SD, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 2002;46:1451-64. ArticlePubMed
- 63. Dannhardt G, Lehr M. Nonsteroidal antiinflammatory agents, XVII: inhibition of bovine cyclooxygenase and 5-lipoxygenase by N-alkyldiphenyl-pyrrolyl acetic and propionic acid derivatives. Arch Pharm (Weinheim) 1993;326:157-62. ArticlePubMed
- 64. Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 2004;36:1187-205. ArticlePubMed
- 65. Wu XD, Xu W, Liu MM, Hu KJ, Sun YY, Yang XF, et al. Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: a meta-analysis of randomized controlled trials. J Surg Oncol 2018;117:1394-404. ArticlePubMedPDF
- 66. Rayes N, Pilarski T, Stockmann M, Bengmark S, Neuhaus P, Seehofer D. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Benef Microbes 2012;3:237-44. ArticlePubMed
- 67. Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014;146:1547-53. ArticlePubMedPMC
- 68. Tanner J, Khan D, Aplin C, Ball J, Thomas M, Bankart J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J Hosp Infect 2009;72:243-50. ArticlePubMed
- 69. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54. ArticlePubMed
- 70. Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 2020;271:1036-47. ArticlePubMed
- 71. Zhang Y, Chen J, Wu J, Chalson H, Merigan L, Mitchell A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg Nutr 2013;2:142-7.ArticlePubMedPMC
- 72. Lytvyn L, Quach K, Banfield L, Johnston BC, Mertz D. Probiotics and synbiotics for the prevention of postoperative infections following abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. J Hosp Infect 2016;92:130-9. ArticlePubMed
- 73. Yang Z, Wu Q, Liu Y, Fan D. Effect of perioperative probiotics and synbiotics on postoperative infections after gastrointestinal surgery: a systematic review with meta-analysis. JPEN J Parenter Enteral Nutr 2017;41:1051-62. ArticlePubMedPDF
- 74. Rammohan A, Sathyanesan J, Rajendran K, Pitchaimuthu A, Perumal SK, Balaraman K, et al. Synbiotics in surgery for chronic pancreatitis: are they truly effective? A single-blind prospective randomized control trial. Ann Surg 2015;262:31-7. ArticlePubMed
- 75. Yokoyama Y, Miyake T, Kokuryo T, Asahara T, Nomoto K, Nagino M. Effect of perioperative synbiotic treatment on bacterial translocation and postoperative infectious complications after pancreatoduodenectomy. Dig Surg 2016;33:220-9. ArticlePubMedPDF
- 76. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018;174:1406-23.e16. ArticlePubMed
- 77. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018;174:1388-405.e21. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

- Postoperative gut dysbiosis and its clinical implications, with an emphasis on probiotic strategies in gastric cancer patients undergoing gastrectomy: a narrative review
Cheong Ah Oh
Ann Clin Nutr Metab.2025; 17(2): 114. CrossRef - Formoterol promotes mitochondrial biogenesis, improves liver regeneration, and suppresses liver injury and inflammation after liver resection in mice with endotoxemia
Amir K Richardson
International Journal of Physiology, Pathophysiology and Pharmacology.2025; 17(4): 131. CrossRef - Probiotics in the Management of Surgery- Induced Diarrhea: Efficacy and Clinical Applications
P. Dhivyaprasath, Gayathri R, Poovitha M, Rabiyath Riswana M, Sabithra P, Susitha R
International Journal of Innovative Science and Research Technology.2024; : 1482. CrossRef
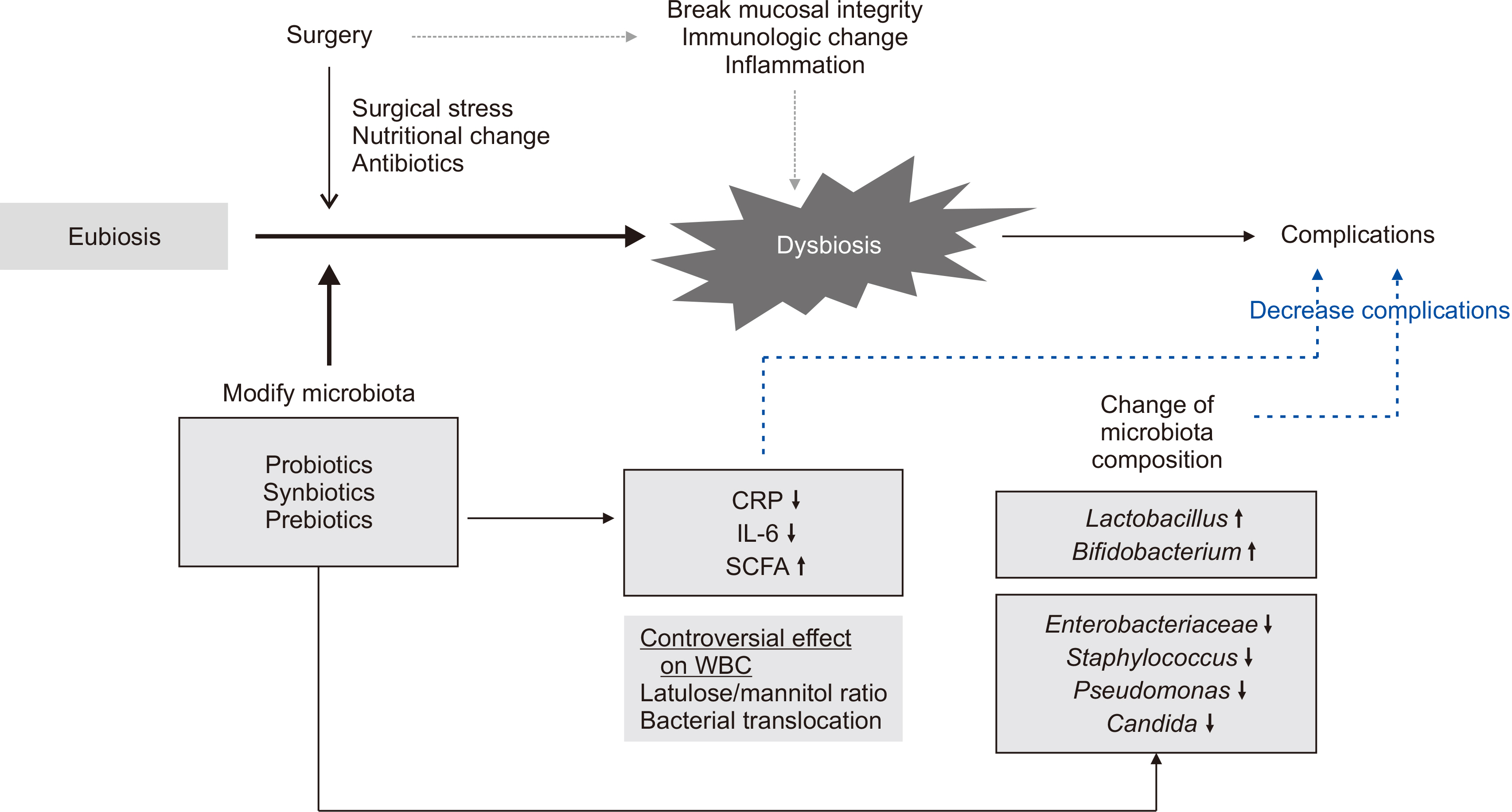
Fig. 1
Probiotics/synbiotics used in clinical trials of patients undergoing abdominal surgery
| Author | Year | Patient number | Indication for surgery | Duration of treatment (day) | Included strains (substrates in synbiotics) |
|---|---|---|---|---|---|
| Synbiotics | |||||
| Rayes et al. [ |
2002 | 90 | Major surgery | 5 |
Lactobacillus plantarum 299v (oat fiber) |
| Kanazawa et al. [ |
2005 | 55 | Biliary cancer | 14 |
Bifidobacterium breve, Lactobacillus casei (galacto-oligosaccharides) |
| Sugawara et al. [ |
2006 | 101 | Liver/biliary surgery | 28 |
Lactobacillus casei Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Rayes et al. [ |
2007 | 89 | Pancreatic surgery | 9 |
Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus plantarum, Lactobacillus paracasei (beta glucan, inulin, pectin, resistant starch) |
| Horvat et al. [ |
2010 | 76 | Colorectal surgery | 3 |
Mixture of 4 Lactobacilli (beta-glucan, inulin, starch, pectin) |
| Eguchi et al. [ |
2011 | 50 | Liver transplantation | Bifidobacterium breve, Lactobacillus casei (galacto-oligosaccharides) | |
| Usami et al. [ |
2011 | 67 | Liver resection | 26 |
Yakult BL seichoyaku (galacto-oligosaccharides) |
| Tanaka et al. [ |
2012 | 64 | Esophagectomy | 22 |
Lactobacillus casei Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Okazaki et al. [ |
2013 | 53 | Upper GI, hepatobiliary, pancreatic cancer | 17 |
Lactobacillus casei Shirota, Bifidobacterium breve Yakult (galacto-oligosaccharides) |
| Sommacal et al. [ |
2015 | 54 | Periampullary tumor | 14 |
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium bifidum (fructo-oligosaccharides) |
| Komatsu et al. [ |
2016 | 379 | Colorectal surgery (laparoscopic) | 9~18 |
Lactobacillus casei Strain Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Yokoyama et al. [ |
2017 | 50 | Pancreatico-duodenectomy | 21 |
Lactobacillus casei Strain Shirota, Bifidobacterium breve strain Yakult (galacto-oligosaccharides) |
| Flesch et al. [ |
2017 | Colorectal cancer | 19 |
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium lactis (fructo-oligosaccharides) | |
| Polakowski et al. [ |
2019 | 77 | Colorectal cancer | 7 |
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium lactis (fructo-oligosaccharide) |
| Park et al. [ |
2020 | 68 | Colorectal cancer | 28 |
Bifidobacterium animalis subsp. lactis HY8002, Lactobacillus casei HY2782, Lactobacillus plantarum HY7712 (lactose, xylitol, maltitol) |
| Probiotics | |||||
| McNaught et al. [ |
2002 | 129 | Major surgery | 14 |
Lactobacillus plantarum 299v |
| Nomura et al. [ |
2007 | 70 | Pancreatic surgery | Variable |
Enterococcus faecalis, Clostridium butyricum, Bacillus mesentericus |
| Liu et al. [ |
2011 | 120 | Colorectal surgery | 16 |
Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum |
| Zhang et al. [ |
2012 | 60 | Colorectal cancer | 3 |
Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis |
| Liu et al. [ |
2013 | 161 | Colorectal cancer | 16 |
Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum |
| Sadahiro et al. [ |
2014 | 310 | Colorectal cancer | 7~23 |
Bifidobacteria |
| Kotzampassi et al. [ |
2015 | 164 | Colorectal cancer | 14 |
Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, Saccharomyces boulardii |
| Yang et al. [ |
2016 | 92 | Colorectal cancer | 12 |
Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis |
| Grąt et al. [ |
2017 | 55 | Liver transplantation | Variable |
Lactobacillus lactis, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium bifidum |
GI = gastrointestinal.
aPostop only; b(/)Pre & postop (duration of preoperative treatment/postoperative treatment).
Changes in microbiota composition after probiotic/synbiotic treatment
| Author | Probiotics/synbiotics | Control | |||
|---|---|---|---|---|---|
| Increase | Decrease | Increase | Decrease | ||
| Synbiotics | |||||
| Kanazawa et al. [ |
Lactobacillus, Bifidobacteriuma Enterococci | Enterobacteriaceae, Pseudomonas, Candida |
|||
| Sugawara et al. [ |
Lactobacillus, Bifidobacterium |
Candida | |||
| Eguchi et al. [ |
No significant difference between groups | ||||
| Usami et al. [ |
Candida | Bacteroidaceae, Bifidobacterium | |||
| Tanaka et al. [ |
Bifidobacterium, Lactobacillus |
Enterobacteriaceae |
|||
| Okazaki et al. [ |
Bifidobacterium |
Enterobacteriaceae, Staphylococcus |
Bifidobacterium | ||
| Komatsu et al. [ |
Clostridium leptum subgroup, Bifidobacterium, Lactobacillus |
Enterobacteriaceae, Staphylococcus, Pseudomonas |
Enterobacteriaceae, Staphylococcus, Pseudomonas, Clostridium difficile |
Clostridium coccoides group, Clostridium leptum subgroup, Bacteroides fragilis group, Bifidobacterium, Prevotella, Lactobacillus |
|
| Yokoyama et al. [ |
Bifidobacterium, Lactobacillus |
Enterobacteriaceae, Pseudomonas |
Pseudomonas, Staphylococcus, Enterobacteriaceae |
||
| Probiotics | |||||
| Liu et al. [ |
Bifidobacterium |
Enterobacteriaceae, Pseudomonas, Candida |
Enterobacteriaceae, Pseudomonas, Candida |
||
| Zhang et al. [ |
Bifidobactrium longum |
Escherichia coli |
Bifidobacterium–Escherichia coli ratio inversion |
||
| Grąt et al. [ |
Enterococcus |
||||
a,bStatistically significant comparing with control group (aP<0.05, bP<0.01).
Comparison of mortality and postoperative infectious complications after abdominal surgery in clinical studies of probiotic/synbiotic treatment
| Author | Infectious complications (%) | Mortality (%) | |||
|---|---|---|---|---|---|
| Probiotics/synbiotics | Control | Probiotics/synbiotics | Control | ||
| Kanazawa et al. [ |
19.0 | 52.2 | 0 | 0 | |
| Rayes et al. [ |
12.5 | 40.0 | 2.5 | 2.5 | |
| Horvat et al. [ |
0 | 5.0 | 0 | 0 | |
| Eguchi et al. [ |
4.0 | 24.0 | 12.0 | 12.0 | |
| Usami et al. [ |
0 | 17.2 | 0 | 0 | |
| Okazaki et al. [ |
24 .0 | 47.8 | 0 | 0 | |
| Sommacal et al. [ |
26.1 | 69.6 | 0 | 10.0 | |
| Komatsu et al. [ |
17.2 | 22.7 | 0 | 0 | |
| Yokoyama et al.[ |
40.9 | 36.4 | 0 | 4.5 | |
| Flesch et al. [ |
2.0 | 21.4 | - | - | |
| Polakowski et al. [ |
2.8 | 18.9 | 0 | 8.1 | |
| Park et al. [ |
6.0 | 28.5 | - | - | |
| McNaught et al. [ |
10.9 | 15.4 | 10.9 | 3.1 | |
| Nomura et al. [ |
23.3 | 52.9 | 0 | 2.9 | |
| Liu et al. [ |
14.0 | 46.0 | 0 | 0 | |
| Zhang et al. [ |
10.0 | 33.3 | - | - | |
| Liu et al. [ |
54.7 | 73.3 | 0 | 0 | |
| Sadahiro et al. [ |
24.0 | 25.3 | - | - | |
| Kotzampassi et al. [ |
19.0 | 28.8 | - | 14.0 | |
| Yang et al. [ |
10.0 | 30.0 | - | - | |
| Grąt et al. [ |
25.0 | 19.2 | - | - | |
- = not available.
GI = gastrointestinal. aPostop only; b(/)Pre & postop (duration of preoperative treatment/postoperative treatment).
a,bStatistically significant comparing with control group (aP<0.05, bP<0.01).
- = not available.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite

