Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 16(2); 2024 > Article
- Original Article Early nutritional support for inpatients reduces admission rates to intensive care units in Korea: a single-center case-control study
-
Hyun Suk Kim1
 , Jae Do Yang1,2
, Jae Do Yang1,2 , Se Wung Han1,2
, Se Wung Han1,2 , Mi Rin Lee1,2
, Mi Rin Lee1,2 , Da-Sol Kim1,3
, Da-Sol Kim1,3 , Sejin Lee1,2
, Sejin Lee1,2 , Seon-Hyeong Kim1
, Seon-Hyeong Kim1 , Chan-Young Kim1,2
, Chan-Young Kim1,2
-
Annals of Clinical Nutrition and Metabolism 2024;16(2):57-65.
DOI: https://doi.org/10.15747/ACNM.2024.16.2.57
Published online: August 1, 2024
1Nutrition Support Team, Jeonbuk National University Hospital, Jeonju, Korea
2Department of Surgery, Jeonbuk National University Hospital, Jeonju, Korea
3Department of Physical Medicine and Rehabilitation, Jeonbuk National University Hospital, Jeonju, Korea
- Corresponding author: Chan-Young Kim, email: happyhill@jbuh.co.kr
© 2024 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,672 Views
- 26 Download
Abstract
-
Purpose Early nutritional support (ENS) for critically ill patients is promoted by many studies. However, there is a lack of data evaluating its necessity in general wards. This study aims to determine the impact of ENS on patients in general wards.
-
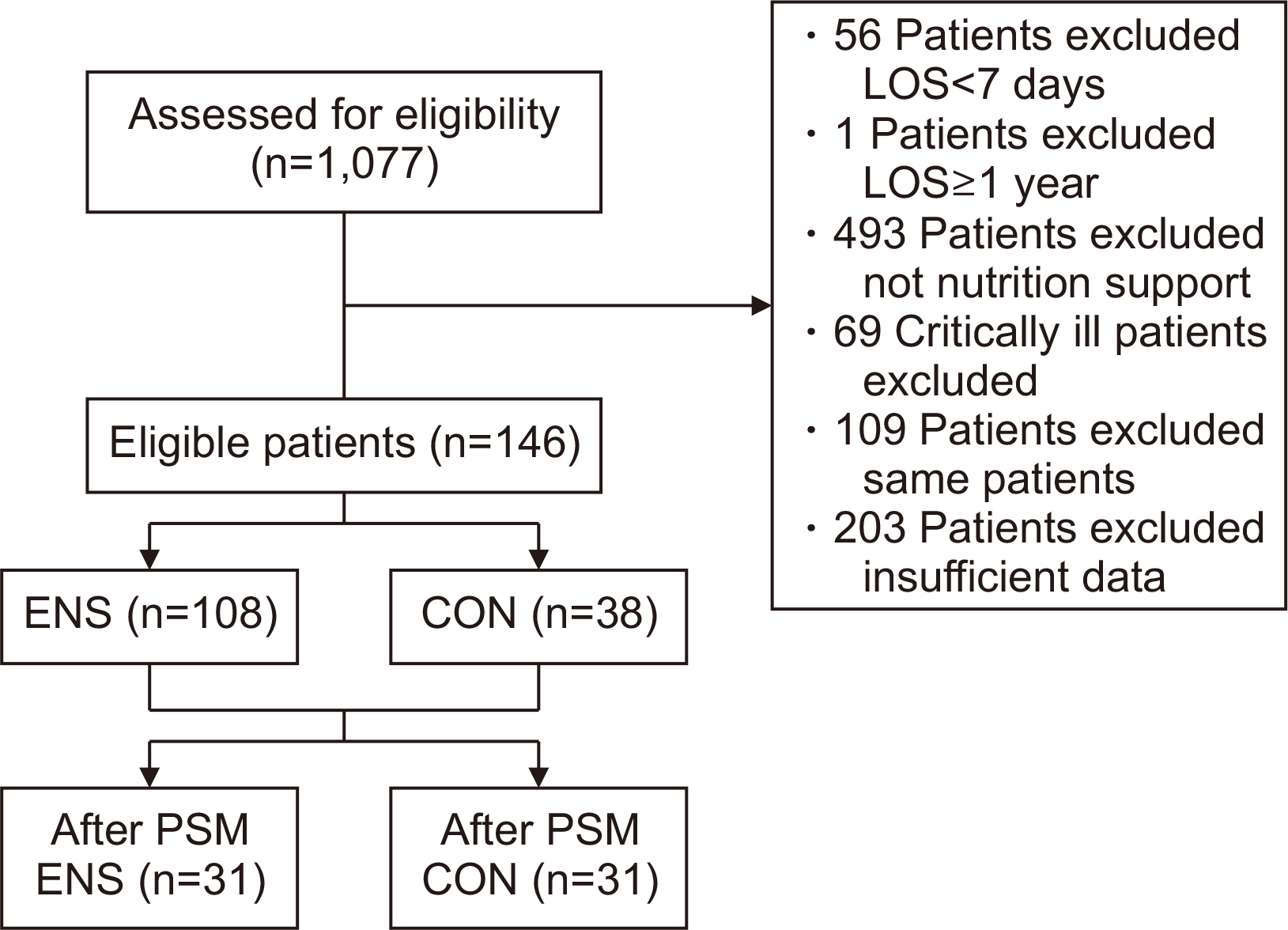
Methods Patients aged 18 and above, admitted to the Jeonbuk National University Hospital in Jeonju from January 2020 to December 2020, who were eligible for nutritional support and hospitalized for at least 7 days were included in the study. We divided the patients into two groups the ENS group, who received nutritional support within 48 hours of admission, and the control group, who received it after 48 hours.
-
Results Among 1,077 patients, 146 met the inclusion criteria. The ENS group (n=38) and the control group (n=108) were compared retrospectively. There was a significant age difference between the two groups (P=0.028). The admission ratio to the intensive care unit (ICU) in the ENS group was significantly lower than that in the control group (10.2% vs. 26.3%, P=0.019). The calorie support rate (%) and protein support rate (%) in the ENS group were significantly higher than in the control group (50.12%±23.30% vs. 38.56%±18.02%, P=0.006; 44.61%±25.07% vs. 32.07%±22.76%, P=0.002, respectively). After propensity score matching, the ENS was significantly associated with ICU low admissions (odds ratio 0.08, 95% confidence interval 0.01–0.69, P=0.022).
-
Conclusion A future multi-center study considering underlying diseases is needed to provide additional scientific evidence to support the effects of ENS.
Graphical abstract
Introduction
Methods
Results
Discussion
Authors’ contribution
Conceptualization: CYK, DSK. Data curation: HSK, MRL. Formal analysis: SL, SWH. Investigation: MRL, DSK, HSK. Methodology: CYK, SWH, SHK. Project administration: SHK, HSK, CYK. Resources: CYK, JDY, SWH, MRL, DSK, SL, SHK, HSK. Supervision: CYK. Validation: JDY, SWH, SHK. Visualization: MRL, DSK, HSK. Writing – original draft: HSK, SL. Writing – review and editing: JDY, SWH, MRL.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.
Supplementary materials
| Variable | ENS (n=108) | CON (n=38) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 64 (59.3) | 21 (55.3) | 0.705b |
| Female | 44 (40.7) | 17 (44.7) | |
| Age (yr) | 77.56±9.79 | 81.66±9.34 | 0.028c |
| Admission type | |||
| Medical | 90 (83.3) | 38 (100) | 0.007b |
| Surgical | 18 (16.7) | 0 (0) | |
| Height (cm) | 160.50±8.90 | 163.18±10.27 | 0.061c |
| Weight (kg) | 50.15±10.47 | 53.00±12.67 | 0.233c |
| BMI (kg/m2) | 19.41±3.56 | 19.86±4.54 | 0.703c |
| BMI range (kg/m2) | |||
| <18.5 (underweight) | 51 (47.2) | 17 (44.7) | 0.677b |
| 18.5–22.9 (normal) | 39 (36.1) | 14 (36.8) | |
| 23.0–24.9 (overweight) | 10 (9.3) | 2 (5.3) | |
| ≥25.0 (obese) | 8 (7.4) | 5 (13.2) | |
| PIBW (%) | 89.57±16.87 | 91.71±21.51 | 0.703c |
| Smoking status | |||
| Former smoker | 25 (23.1) | 9 (23.7) | >0.999b |
| Nonsmoker | 79 (73.1) | 28 (73.7) | |
| Current smoker | 4 (3.7) | 1 (2.6) | |
| Drinking status | |||
| Former | 22 (20.4) | 12 (31.6) | 0.252b |
| None | 78 (72.2) | 22 (57.9) | |
| Current | 8 (7.4) | 4 (10.5) | |
| Nutrition consultationa | |||
| Yes | 26 (24.1) | 6 (15.8) | 0.365b |
| No | 82 (75.9) | 32 (84.2) | |
| Nutrition support access | |||
| EN only | 28 (25.9) | 18 (47.4) | 0.045b |
| PN only | 17 (15.7) | 3 (7.9) | |
| EN & PN | 63 (58.3) | 17 (44.7) | |
| Nutritional status | |||
| No malnutrition | 30 (27.8) | 5 (13.2) | 0.057b |
| Moderate malnutrition | 33 (30.6) | 19 (50.0) | |
| Severe malnutrition | 45 (41.7) | 14 (36.8) |
Values are presented as number (%) or mean±standard deviation.
aConsultation for nutrition support.
bStatistical analysis by χ2-test, cstatistical analysis by Mann–Whitney U-test.
ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; BMI = body mass index; PIBW = percentage ideal body weight; EN = enteral nutrition; PN = parenteral nutrition.
| Variable | ENS (n=108) | CON (n=38) | P-value |
|---|---|---|---|
| Length of NPO (day) | 4.23±6.45 | 6.32±5.69 | <0.001a |
| Length of stay (day) | 22.83±14.89 | 27.42±13.83 | 0.016a,c |
| ICU admission | |||
| Yes | 11 (10.2) | 10 (26.3) | 0.019b,c |
| No | 97 (89.8) | 28 (73.7) | |
| In-hospital mortality | |||
| Yes | 10 (9.3) | 9 (23.7) | 0.029b |
| No | 98 (90.7) | 29 (76.3) |
Values are presented as mean±standard deviation or number (%).
ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; NPO = nil per os; ICU = intensive care unit.
aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by χ2-test; cP<0.05 by χ2-test in internal medicine patients of sub-group.
| Variable | ENS (n=108) | CON (n=38) | P-valuea |
|---|---|---|---|
| Energy (kcal) | |||
| Requirement | 1,388.23±181.52 | 1,434.87±203.64 | 0.113 |
| Order (delivered) | 678.02±294.79 | 546.18±260.58 | 0.016b |
| Total ordered/required calorie ratio (%) | 50.12±23.30 | 38.56±18.02 | 0.006b,c |
| Protein (g) | |||
| Requirement | 59.99±13.43 | 52.00±12.83 | 0.002 |
| Order (delivered) | 25.24±12.39 | 16.74±12.54 | <0.001d |
| Total ordered/required protein ratio (%) | 44.61±25.07 | 32.07±22.76 | 0.002c |
Values are presented as mean±standard deviation.
ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission.
aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by independent t-test; cP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group; dP<0.05 by independent t-test in internal medicine patients of sub-group.
| Variable | ENS (n=108) | CON (n=38) | P-valueb | P-valuec | |||||
|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge | P-valuea | Admission | Discharge | P-valuea | ||||
| Albumin (g/dL) | 3.21±0.66 | 3.10±0.47 | 0.061 | 2.97±0.51 | 2.95±0.42 | 0.879 | 0.098 | 0.093 | |
| TLC (cell/mm3) | 856.18±650.48 | 1,191.21±586.11 | <0.001g | 920.16±670.09 | 1,309.48±828.75 | <0.001g | 0.641 | 0.882 | |
| Hb (g/dL) | 10.71±2.50 | 9.85±1.58 | <0.001g | 10.89±2.83 | 9.15±1.27 | 0.002d,g | 0.717e | 0.015f,h | |
| Hct (%) | 32.19±8.23 | 32.15±21.27 | 0.985d,g | 33.34±8.36 | 28.51±3.91 | 0.003d,g | 0.418e | 0.008h | |
| Glucose (mg/L) | 156.92±77.00 | 152.82±71.55 | 0.258 | 191.82±100.62 | 136.25±42.89 | 0.007g | 0.114 | 0.117f | |
| BUN (mg/dL) | 33.23±32.42 | 29.14±29.55 | 0.172 | 64.61±48.74 | 28.47±22.11 | <0.001g | <0.001h | 0.503 | |
| Creatinine (mg/dL) | 1.63±2.50 | 1.19±1.47 | <0.001g | 2.71±3.42 | 1.18±0.07 | <0.001g | <0.001h | 0.478 | |
| AST (IU/L) | 43.06±40.42 | 57.94±125.30 | 0.537 | 165.82±507.39 | 36.97±25.59 | 0.004g | 0.012h | 0.635 | |
| ALT (IU/L) | 26.80±29.87 | 28.36±40.84 | 0.490 | 64.84±145.14 | 27.75±24.22 | 0.295 | 0.077 | 0.720 | |
| Na (mmol/L) | 136.47±7.68 | 137.52±7.07 | 0.268 | 140.34±11.53 | 137.71±5.11 | 0.359 | 0.118 | 0.878f | |
| K (mmol/L) | 4.07±1.02 | 4.05±0.79 | 0.867 | 4.22±1.03 | 3.87±0.65 | 0.064 | 0.172 | 0.175 | |
| Cl (mmol/L) | 107.73±28.77 | 103.60±17.88 | 0.003g | 107.61±10.75 | 102.74±7.53 | 0.022d,g | 0.260 | 0.837 | |
| Ca (mg/dL) | 8.46±1.06 | 8.73±0.77 | 0.105 | 8.42±0.99 | 8.35±0.77 | 0.713d | 0.749 | 0.027 | |
| P (mg/dL) | 3.44±1.91 | 3.28±1.38 | 0.855 | 4.46±2.41 | 3.06±1.11 | 0.001g | 0.041h | 0.460 | |
| CRP (mg/L) | 87.90±85.11 | 35.81±41.24 | <0.001g | 142.62±70.01 | 54.81±50.05 | <0.001g | <0.001h | 0.013 | |
Values are presented as mean±standard deviation.
ENS = patients who received enteral or parenteral nutritional support within 48 hours of admission; CON = patients who received enteral or parenteral nutritional support 48 hours after admission; TLC = total lymphocyte count; Hb = hemoglobin; Hct = hematocrit; BUN = blood urea nitrogen; AST = aspartate aminotransferase; ALT=alanine aminotransferase; CRP = C-reactive protein.
aStatistical analysis by Wilcoxon signed-rank test within the group; bstatistical analysis by Mann–Whitney U-test at admission between group; cstatistical analysis by Mann–Whitney U-test at discharge between groups; dstatistical analysis by paired t-test; estatistical analysis by independent t-test at admission; fstatistical analysis by independent t-test at discharge; gP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups; hP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups.
| Variable | Model 1 (n=146) | Model 2 (n=146) | Model 3 (n=62) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | aOR (95% CI) | P-value | OR (95% CI) | P-value | |||
| No | 1 | 1 | 1 | |||||
| Yes | 0.32 (0.12–0.82) | 0.018 | 0.14 (0.04–0.53) | 0.004 | 0.08 (0.01–0.69) | 0.022 | ||
Model 1 = univariable logistic regression; Model 2 = multivariable analysis with adjustment of age, admission type, and nutrition support access; Model 3 = logistic regression after propensity score matching.
ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; ICU = intensive care unit; OR = odds ratio; aOR = adjusted odds ratio.
- 1. Skipper A. Agreement on defining malnutrition. JPEN J Parenter Enteral Nutr 2012;36:261-2. ArticlePubMedPDF
- 2. Böhne SEJ, Hiesmayr M, Sulz I, Tarantino S, Wirth R, Volkert D. Recent and current low food intake - prevalence and associated factors in hospital patients from different medical specialities. Eur J Clin Nutr 2022;76:1440-8. ArticlePubMedPMCPDF
- 3. Seol E, Suh YS, Ju DL, Bae HJ, Lee HJ. Characteristics and clinical course of patients who received enteral or parenteral nutrition in tertiary referral hospitals in Korea. J Clin Nutr 2016;8:58-65. Article
- 4. Keller U. Nutritional laboratory markers in malnutrition. J Clin Med 2019;8:775.ArticlePubMedPMC
- 5. Hwang HS, Lee SH, Lee H, Kim KS, Chung SJ, Lee JG. Effects of nutrition consultation on nutritional status in critically ill surgical patients. J Clin Nutr 2015;7:28-34. Article
- 6. Lee JS, Cho MR, Lee GJ. Validation of the developed nutritional screening tool for hospital patients. Korean J Nutr 2010;43:189-96. Article
- 7. Kim BH, Kim H, Kwon O. A comparison of nutritional status by intensive nutritional support in enteral nutrition patients. J Nutr Health 2018;51:132-9. ArticlePDF
- 8. Koga Y, Fujita M, Yagi T, Todani M, Nakahara T, Kawamura Y, et al. Early enteral nutrition is associated with reduced in-hospital mortality from sepsis in patients with sarcopenia. J Crit Care 2018;47:153-8. ArticlePubMed
- 9. Haac B, Henry S, Diaz J, Scalea T, Stein D. Early enteral nutrition is associated with reduced morbidity in critically ill soft tissue patients. Am Surg 2018;84:1003-9. ArticlePubMedPDF
- 10. Pardo E, Lescot T, Preiser JC, Massanet P, Pons A, Jaber S, et al. FRANS Study Group. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care 2023;27:7.ArticlePubMedPMC
- 11. Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr 2023;42:1671-89. ArticlePubMed
- 12. Gressies C, Tribolet P, Schuetz P. Nutrition issues in the general medical ward patient: from general screening to specific diagnosis and individualized treatment. JPEN J Parenter Enteral Nutr 2023;47 Suppl 1:S16-23. ArticlePubMedPDF
- 13. Lee JR, Choi HR. Analysis of risk factors to predict intensive care unit transfer in medical in-patients. J Korean Biol Nurs Sci 2014;16:259-66. Article
- 14. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med 2006;34:2463-78. ArticlePubMed
- 15. Jang JN, Lee YM, Park HJ, Lee HJ. The risk factors related to early readmission to the intensive care unit. J Korean Crit Care Nurs 2019;12:36-45. ArticlePDF
- 16. Wilson RM, Harrison BT, Gibberd RW, Hamilton JD. An analysis of the causes of adverse events from the Quality in Australian Health Care Study. Med J Aust 1999;170:411-5. ArticlePubMedPDF
- 17. Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med 2002;28:1629-34. ArticlePubMedPDF
- 18. Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom--the ACADEMIA study. Resuscitation 2004;62:275-82. ArticlePubMed
- 19. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207-217. ArticlePubMedPMCPDF
- 20. Takagi K, Murotani K, Kamoshita S, Kuroda A. Clinical impact of lipid injectable emulsion in internal medicine inpatients exclusively receiving parenteral nutrition: a propensity score matching analysis from a Japanese medical claims database. BMC Med 2022;20:371.ArticlePubMedPMCPDF
- 21. Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. ArticlePubMedPMC
- 22. Tomino T, Harada N, Toshida K, Tomiyama T, Kosai Y, Kurihara T, et al. Effect of early enteral nutrition on graft loss after living donor liver transplantation: a propensity score matching analysis. Transplant Proc 2023;55:2164-70. ArticlePubMed
- 23. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228-34. Article
- 24. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. ArticlePubMed
- 25. Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health 2011;8:514-27. ArticlePubMedPMC
- 26. Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients 2017;9:829.ArticlePubMedPMC
- 27. Korean Dietetic Association (KDA). Korean Dietetic Association (KDA). Manual of medical nutrition therapy. 4th ed. Vol. 1. KDA, 2022.Article
- 28. Marcason W. Should albumin and prealbumin be used as indicators for malnutrition? J Acad Nutr Diet 2017;117:1144.ArticlePubMed
- 29. Park HS, Lim YS, Kim KA. Park HS, Lim YS, Kim KA. Advanced nutrition and metabolism. Hyoil, 2010.Article
- 30. Lanser L, Fuchs D, Kurz K, Weiss G. Physiology and inflammation driven pathophysiology of iron homeostasis-mechanistic insights into anemia of inflammation and its treatment. Nutrients 2021;13:3732.ArticlePubMedPMC
- 31. Tadlock MD, Hannon M, Davis K, Lancman M, Pamplin J, Shackelford S, et al. Nutritional support using enteral and parenteral methods. Mil Med 2018;183(Suppl 2):153-60. ArticlePubMed
- 32. Jeong HS, Teong CH, Choi YJ, Kim WJ, Lee AR. 2014;Attitudes of medical staff and factors related to nutritional support for patient care in a university hospital. J Clin Nutr 6:37-41. Article
- 33. Choi J, Park E. Different perceptions of clinical nutrition services between doctors and dietitians in the Busan-Gyeongnam area. J Korean Diet Assoc 2013;19:69-81. Article
- 34. Park T, Hong SB, Lim CM, Koh Y. Effect of admission time to the medical intensive care unit on acute critical patient outcomes. Korean J Crit Care Med 2010;25:71-5. Article
- 35. Gearhart AM, Furmanek S, English C, Ramirez J, Cavallazzi R. Predicting the need for ICU admission in community-acquired pneumonia. Respir Med 2019;155:61-5. ArticlePubMed
- 36. Huang Y, Zhang Q, Li P, Chen M, Wang R, Hu J, et al. The prognostic nutritional index predicts all-cause mortality in critically ill patients with acute myocardial infarction. BMC Cardiovasc Disord 2023;23:339.ArticlePubMedPMCPDF
- 37. Gao T, Yu X. Association between nutritional status scores and the 30-day mortality in patients with acute kidney injury: an analysis of MIMIC-III database. BMC Nephrol 2023;24:296.ArticlePubMedPMCPDF
References
Figure & Data
REFERENCES
Citations

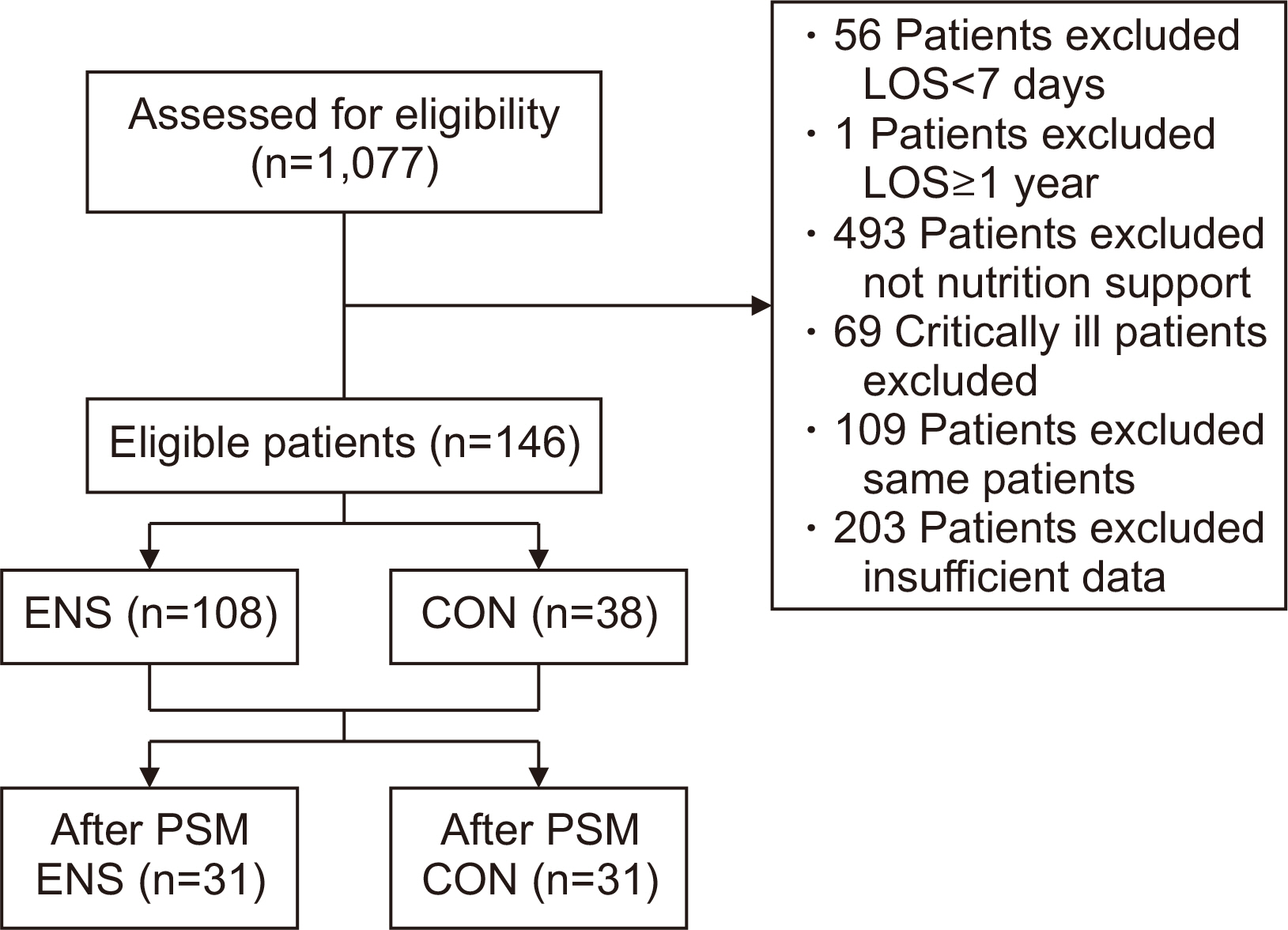
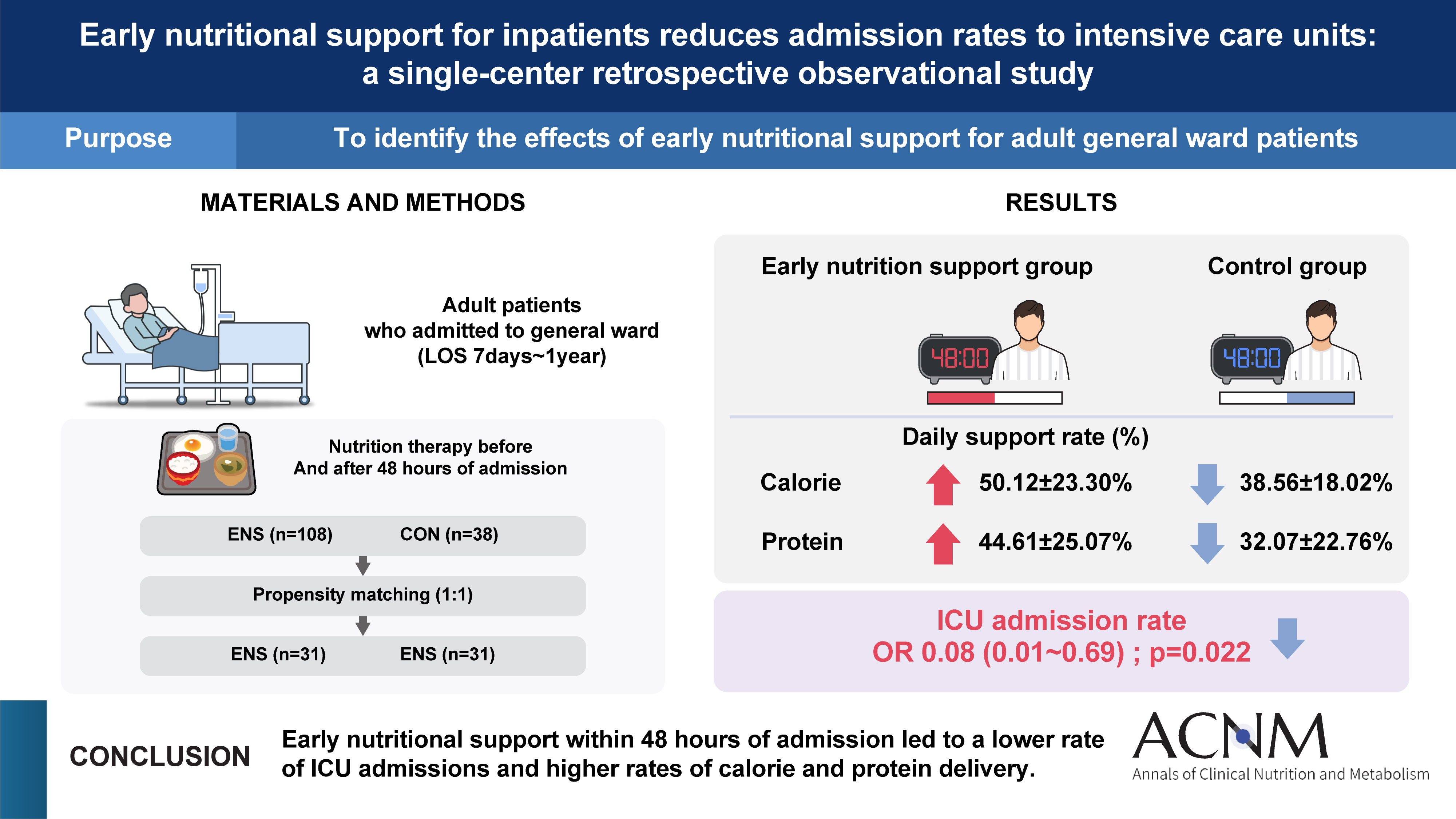
Fig. 1
Graphical abstract
Baseline characteristics of the two groups
| Variable | ENS (n=108) | CON (n=38) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 64 (59.3) | 21 (55.3) | 0.705 |
| Female | 44 (40.7) | 17 (44.7) | |
| Age (yr) | 77.56±9.79 | 81.66±9.34 | 0.028 |
| Admission type | |||
| Medical | 90 (83.3) | 38 (100) | 0.007 |
| Surgical | 18 (16.7) | 0 (0) | |
| Height (cm) | 160.50±8.90 | 163.18±10.27 | 0.061 |
| Weight (kg) | 50.15±10.47 | 53.00±12.67 | 0.233 |
| BMI (kg/m2) | 19.41±3.56 | 19.86±4.54 | 0.703 |
| BMI range (kg/m2) | |||
| <18.5 (underweight) | 51 (47.2) | 17 (44.7) | 0.677 |
| 18.5–22.9 (normal) | 39 (36.1) | 14 (36.8) | |
| 23.0–24.9 (overweight) | 10 (9.3) | 2 (5.3) | |
| ≥25.0 (obese) | 8 (7.4) | 5 (13.2) | |
| PIBW (%) | 89.57±16.87 | 91.71±21.51 | 0.703 |
| Smoking status | |||
| Former smoker | 25 (23.1) | 9 (23.7) | >0.999 |
| Nonsmoker | 79 (73.1) | 28 (73.7) | |
| Current smoker | 4 (3.7) | 1 (2.6) | |
| Drinking status | |||
| Former | 22 (20.4) | 12 (31.6) | 0.252 |
| None | 78 (72.2) | 22 (57.9) | |
| Current | 8 (7.4) | 4 (10.5) | |
| Nutrition consultation |
|||
| Yes | 26 (24.1) | 6 (15.8) | 0.365 |
| No | 82 (75.9) | 32 (84.2) | |
| Nutrition support access | |||
| EN only | 28 (25.9) | 18 (47.4) | 0.045 |
| PN only | 17 (15.7) | 3 (7.9) | |
| EN & PN | 63 (58.3) | 17 (44.7) | |
| Nutritional status | |||
| No malnutrition | 30 (27.8) | 5 (13.2) | 0.057 |
| Moderate malnutrition | 33 (30.6) | 19 (50.0) | |
| Severe malnutrition | 45 (41.7) | 14 (36.8) |
Values are presented as number (%) or mean±standard deviation.
aConsultation for nutrition support.
bStatistical analysis by χ2-test, cstatistical analysis by Mann–Whitney U-test.
ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; BMI = body mass index; PIBW = percentage ideal body weight; EN = enteral nutrition; PN = parenteral nutrition.
Outcomes of patients in the two groups
| Variable | ENS (n=108) | CON (n=38) | P-value |
|---|---|---|---|
| Length of NPO (day) | 4.23±6.45 | 6.32±5.69 | <0.001 |
| Length of stay (day) | 22.83±14.89 | 27.42±13.83 | 0.016 |
| ICU admission | |||
| Yes | 11 (10.2) | 10 (26.3) | 0.019 |
| No | 97 (89.8) | 28 (73.7) | |
| In-hospital mortality | |||
| Yes | 10 (9.3) | 9 (23.7) | 0.029 |
| No | 98 (90.7) | 29 (76.3) |
Values are presented as mean±standard deviation or number (%).
ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; NPO = nil per os; ICU = intensive care unit.
aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by χ2-test; cP<0.05 by χ2-test in internal medicine patients of sub-group.
Daily required and delivered nutrition amount in the two groups
| Variable | ENS (n=108) | CON (n=38) | P-value |
|---|---|---|---|
| Energy (kcal) | |||
| Requirement | 1,388.23±181.52 | 1,434.87±203.64 | 0.113 |
| Order (delivered) | 678.02±294.79 | 546.18±260.58 | 0.016 |
| Total ordered/required calorie ratio (%) | 50.12±23.30 | 38.56±18.02 | 0.006 |
| Protein (g) | |||
| Requirement | 59.99±13.43 | 52.00±12.83 | 0.002 |
| Order (delivered) | 25.24±12.39 | 16.74±12.54 | <0.001 |
| Total ordered/required protein ratio (%) | 44.61±25.07 | 32.07±22.76 | 0.002 |
Values are presented as mean±standard deviation.
ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission.
aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by independent t-test; cP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group; dP<0.05 by independent t-test in internal medicine patients of sub-group.
Laboratory data in the two groups
| Variable | ENS (n=108) | CON (n=38) | P-value |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge | P-value |
Admission | Discharge | P-value |
||||
| Albumin (g/dL) | 3.21±0.66 | 3.10±0.47 | 0.061 | 2.97±0.51 | 2.95±0.42 | 0.879 | 0.098 | 0.093 | |
| TLC (cell/mm3) | 856.18±650.48 | 1,191.21±586.11 | <0.001 |
920.16±670.09 | 1,309.48±828.75 | <0.001 |
0.641 | 0.882 | |
| Hb (g/dL) | 10.71±2.50 | 9.85±1.58 | <0.001 |
10.89±2.83 | 9.15±1.27 | 0.002 |
0.717 |
0.015 |
|
| Hct (%) | 32.19±8.23 | 32.15±21.27 | 0.985 |
33.34±8.36 | 28.51±3.91 | 0.003 |
0.418 |
0.008 |
|
| Glucose (mg/L) | 156.92±77.00 | 152.82±71.55 | 0.258 | 191.82±100.62 | 136.25±42.89 | 0.007 |
0.114 | 0.117 |
|
| BUN (mg/dL) | 33.23±32.42 | 29.14±29.55 | 0.172 | 64.61±48.74 | 28.47±22.11 | <0.001 |
<0.001 |
0.503 | |
| Creatinine (mg/dL) | 1.63±2.50 | 1.19±1.47 | <0.001 |
2.71±3.42 | 1.18±0.07 | <0.001 |
<0.001 |
0.478 | |
| AST (IU/L) | 43.06±40.42 | 57.94±125.30 | 0.537 | 165.82±507.39 | 36.97±25.59 | 0.004 |
0.012 |
0.635 | |
| ALT (IU/L) | 26.80±29.87 | 28.36±40.84 | 0.490 | 64.84±145.14 | 27.75±24.22 | 0.295 | 0.077 | 0.720 | |
| Na (mmol/L) | 136.47±7.68 | 137.52±7.07 | 0.268 | 140.34±11.53 | 137.71±5.11 | 0.359 | 0.118 | 0.878 |
|
| K (mmol/L) | 4.07±1.02 | 4.05±0.79 | 0.867 | 4.22±1.03 | 3.87±0.65 | 0.064 | 0.172 | 0.175 | |
| Cl (mmol/L) | 107.73±28.77 | 103.60±17.88 | 0.003 |
107.61±10.75 | 102.74±7.53 | 0.022 |
0.260 | 0.837 | |
| Ca (mg/dL) | 8.46±1.06 | 8.73±0.77 | 0.105 | 8.42±0.99 | 8.35±0.77 | 0.713 |
0.749 | 0.027 | |
| P (mg/dL) | 3.44±1.91 | 3.28±1.38 | 0.855 | 4.46±2.41 | 3.06±1.11 | 0.001 |
0.041 |
0.460 | |
| CRP (mg/L) | 87.90±85.11 | 35.81±41.24 | <0.001 |
142.62±70.01 | 54.81±50.05 | <0.001 |
<0.001 |
0.013 | |
Values are presented as mean±standard deviation.
ENS = patients who received enteral or parenteral nutritional support within 48 hours of admission; CON = patients who received enteral or parenteral nutritional support 48 hours after admission; TLC = total lymphocyte count; Hb = hemoglobin; Hct = hematocrit; BUN = blood urea nitrogen; AST = aspartate aminotransferase; ALT=alanine aminotransferase; CRP = C-reactive protein.
aStatistical analysis by Wilcoxon signed-rank test within the group; bstatistical analysis by Mann–Whitney U-test at admission between group; cstatistical analysis by Mann–Whitney U-test at discharge between groups; dstatistical analysis by paired t-test; estatistical analysis by independent t-test at admission; fstatistical analysis by independent t-test at discharge; gP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups; hP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups.
Effect of ENS on ICU admission for general ward patients
| Variable | Model 1 (n=146) | Model 2 (n=146) | Model 3 (n=62) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | aOR (95% CI) | P-value | OR (95% CI) | P-value | |||
| No | 1 | 1 | 1 | |||||
| Yes | 0.32 (0.12–0.82) | 0.018 | 0.14 (0.04–0.53) | 0.004 | 0.08 (0.01–0.69) | 0.022 | ||
Model 1 = univariable logistic regression; Model 2 = multivariable analysis with adjustment of age, admission type, and nutrition support access; Model 3 = logistic regression after propensity score matching.
ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; ICU = intensive care unit; OR = odds ratio; aOR = adjusted odds ratio.
Values are presented as number (%) or mean±standard deviation. aConsultation for nutrition support. bStatistical analysis by χ2-test, cstatistical analysis by Mann–Whitney U-test. ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; BMI = body mass index; PIBW = percentage ideal body weight; EN = enteral nutrition; PN = parenteral nutrition.
Values are presented as mean±standard deviation or number (%). ENS = patients who received enteral or parenteral nutrition within 48 hours after admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission; NPO = nil per os; ICU = intensive care unit. aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by χ2-test; cP<0.05 by χ2-test in internal medicine patients of sub-group.
Values are presented as mean±standard deviation. ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; CON = patients who received enteral or parenteral nutrition support 48 hours after admission. aStatistical analysis by Mann–Whitney U-test; bstatistical analysis by independent t-test; cP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group; dP<0.05 by independent t-test in internal medicine patients of sub-group.
Values are presented as mean±standard deviation. ENS = patients who received enteral or parenteral nutritional support within 48 hours of admission; CON = patients who received enteral or parenteral nutritional support 48 hours after admission; TLC = total lymphocyte count; Hb = hemoglobin; Hct = hematocrit; BUN = blood urea nitrogen; AST = aspartate aminotransferase; ALT=alanine aminotransferase; CRP = C-reactive protein. aStatistical analysis by Wilcoxon signed-rank test within the group; bstatistical analysis by Mann–Whitney U-test at admission between group; cstatistical analysis by Mann–Whitney U-test at discharge between groups; dstatistical analysis by paired t-test; estatistical analysis by independent t-test at admission; fstatistical analysis by independent t-test at discharge; gP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups; hP<0.05 by Mann–Whitney U-test in internal medicine patients of sub-group between groups.
Model 1 = univariable logistic regression; Model 2 = multivariable analysis with adjustment of age, admission type, and nutrition support access; Model 3 = logistic regression after propensity score matching. ENS = patients who received enteral or parenteral nutrition within 48 hours of admission; ICU = intensive care unit; OR = odds ratio; aOR = adjusted odds ratio.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN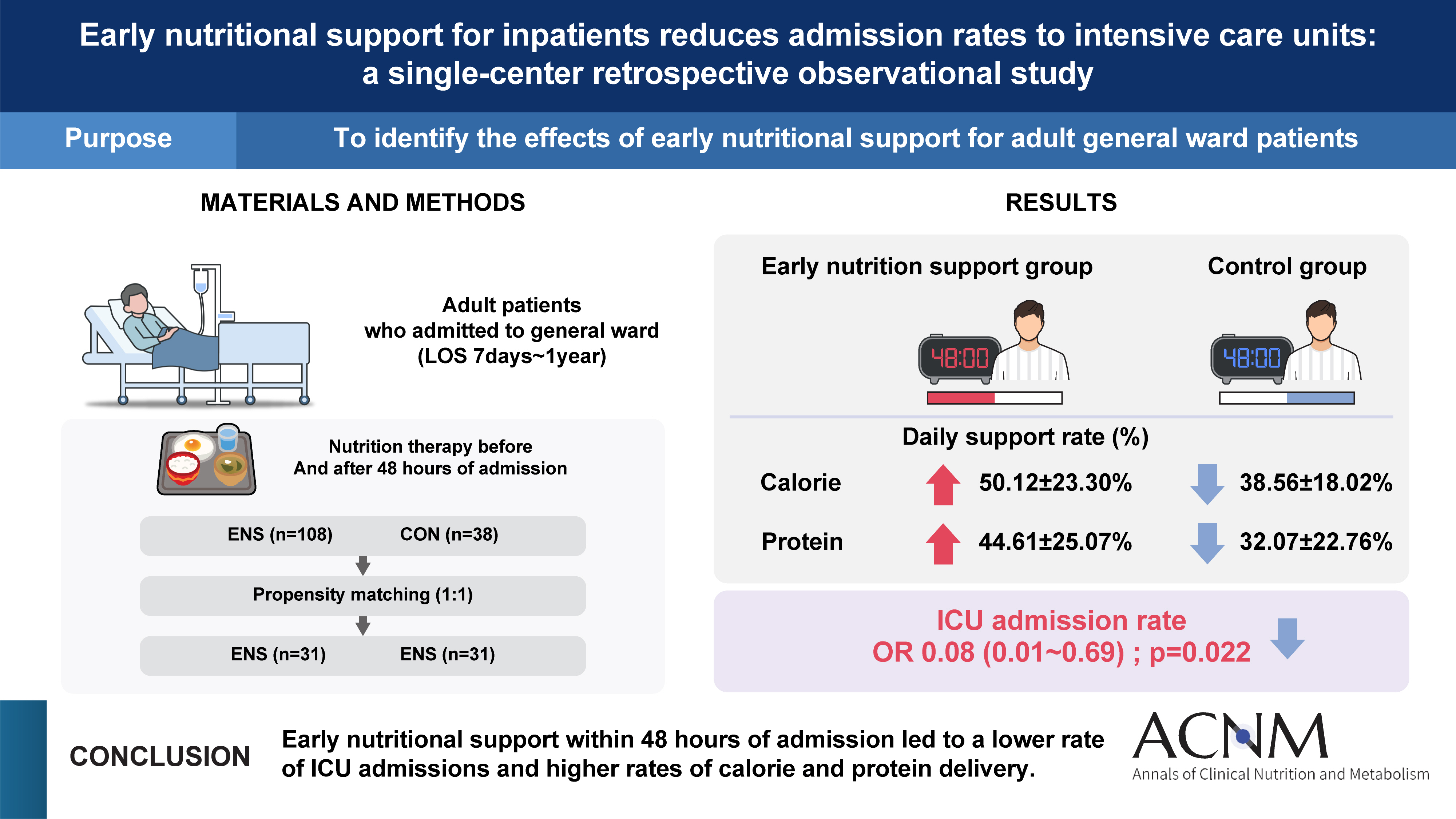
 Cite
Cite

