Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 16(3); 2024 > Article
- Guideline Nutritional support for critically ill patients by the Korean Society for Parenteral and Enteral Nutrition — part I: a clinical practice guideline
-
Seung Hwan Lee1
 , Jae Gil Lee2
, Jae Gil Lee2 , Min Kwan Kwon3
, Min Kwan Kwon3 , Jiyeon Kim4
, Jiyeon Kim4 , Mina Kim5
, Mina Kim5 , Jeongyun Park6
, Jeongyun Park6 , Jee Young Lee7
, Jee Young Lee7 , Ye Won Sung8
, Ye Won Sung8 , Bomi Kim9
, Bomi Kim9 , Seong Eun Kim10
, Seong Eun Kim10 , Ji Yoon Cho11
, Ji Yoon Cho11 , A Young Lim12
, A Young Lim12 , In Gyu Kwon13
, In Gyu Kwon13 , Miyoung Choi14
, Miyoung Choi14 , KSPEN Guideline Committee
, KSPEN Guideline Committee -
Annals of Clinical Nutrition and Metabolism 2024;16(3):89-111.
DOI: https://doi.org/10.15747/ACNM.2024.16.3.89
Published online: December 1, 2024
1Department of Traumatology, Gachon University College of Medicine, Incheon, Korea
2Department of Surgery, Ewha Womans University Mokdong Hospital, Seoul, Korea
3Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
4Department of Clinical Nutrition, National Cancer Center, Goyang, Korea
5Department of Nursing, Inha University Hospital, Incheon, Korea
6Department of Clinical Nursing, University of Ulsan, Ulsan, Korea
7Department of Nursing, Kosin University Gospel Hospital, Busan, Korea
8Department of Pharmacy, Chungnam National University Hospital, Daejeon, Korea
9Department of Pharmacy, Seoul National University Hospital, Seoul, Korea
10Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea
11Department of Pharmacy, Daegu Fatima Hospital, Daegu, Korea
12Department of Clinical Nutrition, Seoul National University Bundang Hospital, Seongnam, Korea
13Department of Surgery, Yonsei University Gangnam Severance Hospital, Seoul, Korea
14Division of Healthcare Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- Corresponding author: Jae Gil Lee, email: jakii71@gmail.com
© 2024 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 24,936 Views
- 634 Download
- 1 Crossref
Abstract
-
Purpose Nutritional support for adult critically ill patients is essential due to the high risk of malnutrition, which can lead to severe complications. This paper aims to develop evidence-based guidelines to optimize nutritional support in intensive care units (ICUs).
-
Methods The Grading Recommendations, Assessment, Development and Evaluation process was used to develop and summarize the evidence on which the recommendations were based. Clinical outcomes were assessed for seven key questions.
-
Results We recommend the following (1) initiate enteral nutrition (EN) within 48 hours after treatment as it is associated with improved outcomes, including reduced infection rates and shorter ICU stays; (2) early EN is preferred over early parenteral nutrition due to better clinical outcomes; (3) the use of supplementary parenteral nutrition to meet energy targets during the first week of ICU admission in patients receiving early EN is conditionally recommended based on patient-specific needs; (4) limited caloric support should be supplied to prevent overfeeding and related complications, particularly in the early phase of critical illness; (5) higher protein intake is suggested to improve clinical outcomes, such as muscle preservation and overall recovery; (6) additional enteral or parenteral glutamine is conditionally recommended against due to the lack of significant benefit and potential harm; and (7) fish oil-containing lipid emulsions is conditionally recommended due to their potential to enhance clinical outcomes, including reduced infection rates and shorter ICU stays.
-
Conclusion These evidence-based recommendations can improve clinical outcomes and support healthcare providers in making informed decisions about nutritional interventions in the ICU.
Purpose
Guideline limitations
Target population
Inclusion criteria
Exclusion criteria
Target audience
Scope
Methods
Literature search
Data acquisition
Evidence quality assessment
Statistical analysis
Results
Conclusions
Supplementary materials
Acknowledgments
Authors’ contribution
Conceptualization: all authors. Data curation: all authors. Formal analysis: all authors. Funding acquisition: JGL, JP. Investigation: SHL, JGL, JP. Methodology: MC. Project administration: JGL, JP. Resources: all authors. Software: all authors. Supervision: SHL, JGL, JP. Validation: SHL, JGL. Visualization: all authors. Writing – original draft: all authors. Writing – review & editing: all authors.
Conflict of interest
These clinical guidelines were developed with financial support from the KSPEN. This financial support did not directly or potentially influence the content or development of the guidelines. Literature searches were supported by the National Evidence-based Healthcare Collaborating Agency (NECA), and this support also did not directly or potentially influence the development of the guidelines. None of the members of the development committee had financial, non-financial, or other conflicts of interest that could affect the guidelines.
Jae Gil Lee is an editorial board member of the journal, but was not involved in the review process of this manuscript. Otherwise, there is no conflict of interest to disclose.
Funding
These clinical guidelines were developed with financial support from the KSPEN.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. All relevant data and materials are included within the manuscript and its supplementary information files.
| Consensus strength | % agreement |
|---|---|
| Strong consensus | >90 |
| Consensus | >75–90 |
| Majority consensus | >50–75 |
| No consensus | <50 |
| Outcomes | Authors | Study year | Findings | Reference |
|---|---|---|---|---|
| Mortality | Heyland et al. | 2003 | RR=0.52, 95% CI=0.25 to 1.08, P=0.08 | [28] |
| Taylor et al. | 2016 | RR=0.70, 95% CI=0.49 to 1.00, P=0.05 | [23] | |
| Reintam Blaser et al. | 2017 | RR=0.76, 95% CI=0.52 to 1.11, P=0.149 | [22] | |
| Pu et al. | 2018 | OR=0.36, 95% CI=0.18 to 0.72, P=0.003 | [24] | |
| Kim et al. | 2021 | Mortality hazard ratio=0.413, 95% CI=0.174 to 0.984, P=0.046 | [30] | |
| Park et al. | 2016 | EN≤1.5 days 11.8% vs. EN>1.5 days 42.9%, P=0.018 | [31] | |
| Choi et al. | 2023 | EEN=14.1% vs. DEN 33.1%, P<0.001 | [32] | |
| Infections | Heyland et al. | 2003 | RR=0.66, 95% CI=0.36 to 1.22, P=0.19 | [28] |
| Taylor et al. | 2016 | RR=0.74, 95% CI=0.58 to 0.93, P=0.01 | [23] | |
| Reintam Blaser et al. | 2017 | RR=0.64, 95% CI=0.46 to 0.90, P=0.010 | [22] | |
| Pu et al. | 2018 | OR=0.23, 95% CI=0.11 to 0.48, P<0.0001 | [24] | |
| Singer et al. | 2023 | RR=0.76, 95% CI=0.59 to 0.97, P<0.03 | [5] | |
| Length of stay | Marik and Zaloga | 2001 | MD=2.2 days, 95% CI=0.81 to 3.63 days, P=0.001 | [25] |
| Pu et al. | 2018 | −15.31 days, 95% CI=−20.43 to −10.20, P<0.00001 | [24] |
- 1. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277-316. ArticlePubMed
- 2. Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition 1996;12:23-9. ArticlePubMed
- 3. Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med 2009;361:1088-97. ArticlePubMedPMC
- 4. Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2022;46:12-41. ArticlePubMedPDF
- 5. Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr 2023;42:1671-89. ArticlePubMed
- 6. Schneider SM, Veyres P, Pivot X, Soummer AM, Jambou P, Filippi J, et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr 2004;92:105-11. ArticlePubMed
- 7. Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, et al. Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 2008;300:2731-41. ArticlePubMed
- 8. Wilmore DW. Catabolic illness. Strategies for enhancing recovery. N Engl J Med 1991;325:695-702. ArticlePubMed
- 9. Burnham EL, Moss M, Ziegler TR. Myopathies in critical illness: characterization and nutritional aspects. J Nutr 2005;135:1818S-23S. ArticlePubMed
- 10. Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab 2008;294:E1-9. ArticlePubMed
- 11. Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 1987;205:288-94. ArticlePubMedPMC
- 12. Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma 1987;27:262-6. ArticlePubMed
- 13. O'Brien JM Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med 2006;34:738-44. ArticlePubMedPMC
- 14. Quenot JP, Plantefeve G, Baudel JL, Camilatto I, Bertholet E, Cailliod R, et al. Bedside adherence to clinical practice guidelines for enteral nutrition in critically ill patients receiving mechanical ventilation: a prospective, multi-centre, observational study. Crit Care 2010;14:R37.ArticlePubMedPMCPDF
- 15. Parent B, Shelton M, Nordlund M, Aarabi S, O'Keefe G. Parenteral nutrition utilization after implementation of multidisciplinary nutrition support team oversight: a prospective cohort study. JPEN J Parenter Enteral Nutr 2016;40:1151-7. ArticlePubMed
- 16. Seol EM, Suh YS, Ju DL, Bae HJ, Kim E, Lee HJ. Nutrition support team reconsultation during nutrition therapy in Korea. JPEN J Parenter Enteral Nutr 2021;45:357-65. ArticlePubMedPDF
- 17. Braun K, Utech A, Velez ME, Walker R. Parenteral nutrition electrolyte abnormalities and associated factors before and after nutrition support team initiation. JPEN J Parenter Enteral Nutr 2018;42:387-92. ArticlePubMedPDF
- 18. Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004;125:1446-57. ArticlePubMed
- 19. Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ 1999;318:527-30. ArticlePubMedPMC
- 20. National Evidence-based Healthcare Collaborating Agency (NECA), Korean Academy of Medical Sciences. Handbook for Clinical Practice Guideline Developer Version 2.0. NECA; 2022.Article
- 21. Fuentes Padilla P, Martínez G, Vernooij RW, Urrútia G, Roqué I Figuls M, Bonfill Cosp X. Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev 2019;2019:CD012340. ArticlePubMedPMC
- 22. Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, et al. ESICM Working Group on Gastrointestinal Function. 2017;Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 43:380-98. ArticlePubMedPMCPDF
- 23. Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Society of Critical Care Medicine; American Society of Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390-438. ArticlePubMed
- 24. Pu H, Doig GS, Heighes PT, Allingstrup MJ. Early enteral nutrition reduces mortality and improves other key outcomes in patients with major burn injury: a meta-analysis of randomized controlled trials. Crit Care Med 2018;46:2036-42. ArticlePubMed
- 25. Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 2001;29:2264-70. ArticlePubMed
- 26. Ortiz-Reyes L, Patel JJ, Jiang X, Coz Yataco A, Day AG, Shah F, et al. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: a nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit Care 2022;26:173.ArticlePubMedPMCPDF
- 27. Doig GS, Chevrou-Séverac H, Simpson F. Early enteral nutrition in critical illness: a full economic analysis using US costs. Clinicoecon Outcomes Res 2013;5:429-36. ArticlePubMedPMC
- 28. Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 2003;27:355-73. ArticlePubMed
- 29. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. ArticlePubMed
- 30. Kim S, Jeong SK, Hwang J, Kim JH, Shin JS, Shin HJ. Early enteral nutrition and factors related to in-hospital mortality in people on extracorporeal membrane oxygenation. Nutrition 2021;89:111222. ArticlePubMed
- 31. Park KW, Son HR, Kim JH, Kim MH, Choi EJ. 2016;The effects of early enteral nutrition in patients: a role of nutrition support team. J Clin Nutr 8:66-70. Article
- 32. Choi YK, Kim HJ, Ahn J, Ryu JA. Impact of early nutrition and feeding route on clinical outcomes of neurocritically ill patients. PLoS One 2023;18:e0283593. ArticlePubMedPMC
- 33. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. 2021;Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 49:e1063-143.ArticlePubMed
- 34. Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Iba T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J Intensive Care 2021;9:53.ArticlePubMedPMC
- 35. Mehta Y, Sunavala JD, Zirpe K, Tyagi N, Garg S, Sinha S, et al. Practice guidelines for nutrition in critically ill patients: a relook for Indian scenario. Indian J Crit Care Med 2018;22:263-73. ArticlePubMedPMC
- 36. Rapp RP, Young B, Twyman D, Bivins BA, Haack D, Tibbs PA, et al. The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg 1983;58:906-12. ArticlePubMed
- 37. Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma 1986;26:882-91. ArticlePubMed
- 38. Young B, Ott L, Twyman D, Norton J, Rapp R, Tibbs P, et al. The effect of nutritional support on outcome from severe head injury. J Neurosurg 1987;67:668-76. ArticlePubMed
- 39. Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma 1989;29:916-23. ArticlePubMed
- 40. Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 1992;215:503-13. ArticlePubMedPMC
- 41. Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z. Gut failure--predictor of or contributor to mortality in mechanically ventilated blunt trauma patients? J Trauma 1994;37:30-4. ArticlePubMed
- 42. Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R, et al. Enteral versus parenteral nutrition after severe closed head injury. J Trauma 1994;37:459-68. ArticlePubMed
- 43. Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 1995;152(5 Pt 1):1545-8. ArticlePubMed
- 44. Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. 1997;Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 84:1665-9. ArticlePubMedPDF
- 45. Woodcock NP, Zeigler D, Palmer MD, Buckley P, Mitchell CJ, MacFie J. Enteral versus parenteral nutrition: a pragmatic study. Nutrition 2001;17:1-12. ArticlePubMed
- 46. Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med 2003;29:834-40. ArticlePubMedPDF
- 47. Radrizzani D, Bertolini G, Facchini R, Simini B, Bruzzone P, Zanforlin G, et al. Early enteral immunonutrition vs. parenteral nutrition in critically ill patients without severe sepsis: a randomized clinical trial. Intensive Care Med 2006;32:1191-8. ArticlePubMedPDF
- 48. Casas M, Mora J, Fort E, Aracil C, Busquets D, Galter S, et al. [Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis]. Rev Esp Enferm Dig 2007;99:264-9; Spanish. ArticlePubMed
- 49. Altintas ND, Aydin K, Türkoğlu MA, Abbasoğlu O, Topeli A. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutr Clin Pract 2011;26:322-9. ArticlePubMedPDF
- 50. Justo Meirelles CM, de Aguilar-Nascimento JE. Enteral or parenteral nutrition in traumatic brain injury: a prospective randomised trial. Nutr Hosp 2011;26:1120-4.ArticlePubMed
- 51. Wang G, Wen J, Xu L, Zhou S, Gong M, Wen P, et al. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J Surg Res 2013;183:592-7. ArticlePubMed
- 52. Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol 2013;19:917-22. ArticlePubMedPMC
- 53. Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673-84. ArticlePubMed
- 54. Fan M, Wang Q, Fang W, Jiang Y, Li L, Sun P, et al. Early enteral combined with parenteral nutrition treatment for severe traumatic brain injury: effects on immune function, nutritional status and outcomes. Chin Med Sci J 2016;31:213-20. ArticlePubMed
- 55. Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. NUTRIREA-2 Trial Investigators; Clinical Research in Intensive Care and Sepsis (CRICS) group. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133-43. ArticlePubMed
- 56. Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117.ArticlePubMedPMC
- 57. Zhang G, Zhang K, Cui W, Hong Y, Zhang Z. The effect of enteral versus parenteral nutrition for critically ill patients: a systematic review and meta-analysis. J Clin Anesth 2018;51:62-92. ArticlePubMed
- 58. Berger MM, Burgos R, Casaer MP, De Robertis E, Delgado JCL, Fraipont V, et al. Clinical nutrition issues in 2022: what is missing to trust supplemental parenteral nutrition (SPN) in ICU patients? Crit Care 2022;26:271.ArticlePubMedPMCPDF
- 59. Alsharif DJ, Alsharif FJ, Aljuraiban GS, Abulmeaty MMA. Effect of supplemental parenteral nutrition versus enteral nutrition alone on clinical outcomes in critically ill adult patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2020;12:2968.ArticlePubMedPMC
- 60. Hill A, Heyland DK, Ortiz Reyes LA, Laaf E, Wendt S, Elke G, et al. Combination of enteral and parenteral nutrition in the acute phase of critical illness: an updated systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2022;46:395-410. ArticlePubMedPDF
- 61. Bauer P, Charpentier C, Bouchet C, Nace L, Raffy F, Gaconnet N. Parenteral with enteral nutrition in the critically ill. Intensive Care Med 2000;26:893-900. ArticlePubMedPDF
- 62. Berger MM, Pantet O, Jacquelin-Ravel N, Charrière M, Schmidt S, Becce F, et al. Supplemental parenteral nutrition improves immunity with unchanged carbohydrate and protein metabolism in critically ill patients: the SPN2 randomized tracer study. Clin Nutr 2019;38:2408-16. ArticlePubMed
- 63. Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381:385-93. ArticlePubMed
- 64. Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med 2017;43:1637-47. ArticlePubMedPDF
- 65. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506-17. ArticlePubMed
- 66. Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130-8. ArticlePubMed
- 67. Ridley EJ, Davies AR, Parke R, Bailey M, McArthur C, Gillanders L, et al. Supplemental Parenteral Nutrition Clinical Investigators. Supplemental parenteral nutrition versus usual care in critically ill adults: a pilot randomized controlled study. Crit Care 2018;22:12.ArticlePubMedPMC
- 68. Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, et al. Intensive nutrition in acute lung injury: a clinical trial (INTACT). JPEN J Parenter Enteral Nutr 2015;39:13-20. ArticlePubMed
- 69. Wischmeyer PE, Hasselmann M, Kummerlen C, Kozar R, Kutsogiannis DJ, Karvellas CJ, et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: the TOP-UP pilot trial. Crit Care 2017;21:142.ArticlePubMedPMCPDF
- 70. Al-Dorzi HM, Albarrak A, Ferwana M, Murad MH, Arabi YM. Lower versus higher dose of enteral caloric intake in adult critically ill patients: a systematic review and meta-analysis. Crit Care 2016;20:358.ArticlePubMedPMCPDF
- 71. Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 2011;39:967-74. ArticlePubMedPMC
- 72. Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307:795-803. ArticlePubMedPMC
- 73. Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr 2014;100:1337-43. ArticlePubMedPMC
- 74. Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. JPEN J Parenter Enteral Nutr 2016;40:242-9. ArticlePubMed
- 75. Reignier J, Plantefeve G, Mira JP, Argaud L, Asfar P, Aissaoui N, et al. NUTRIREA-3 Trial Investigators; Clinical Research in Intensive Care; Sepsis (CRICS-TRIGGERSEP)Group. Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med 2023;11:602-12. ArticlePubMed
- 76. Singer P, De Waele E, Sanchez C, Ruiz Santana S, Montejo JC, Laterre PF, et al. TICACOS international: a multi-center, randomized, prospective controlled study comparing tight calorie control versus liberal calorie administration study. Clin Nutr 2021;40:380-7. ArticlePubMed
- 77. Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011;93:569-77. ArticlePubMed
- 78. Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. PermiT Trial Group. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med 2015;372:2398-408. ArticlePubMed
- 79. Charles EJ, Kane WJ, Willcutts KF, O'Donnell KB, Petroze RT, Sawyer RG. Hypoenergetic feeding does not improve outcomes in critically ill patients with premorbid obesity: a post hoc analysis of a randomized controlled trial. Nutr Res 2020;74:71-7. ArticlePubMed
- 80. Rugeles S, Villarraga-Angulo LG, Ariza-Gutiérrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D. High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care 2016;35:110-4. ArticlePubMed
- 81. Doig GS, Simpson F, Bellomo R, Heighes PT, Sweetman EA, Chesher D, et al. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 2015;41:1197-208. ArticlePubMedPDF
- 82. Ferrie S, Allman-Farinelli M, Daley M, Smith K. Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. JPEN J Parenter Enteral Nutr 2016;40:795-805. ArticlePubMed
- 83. Fetterplace K, Deane AM, Tierney A, Beach LJ, Knight LD, Presneill J, et al. Targeted full energy and protein delivery in critically ill patients: a pilot randomized controlled trial (FEED Trial). JPEN J Parenter Enteral Nutr 2018;42:1252-62. ArticlePubMedPDF
- 84. van Zanten ARH, Petit L, De Waele J, Kieft H, de Wilde J, van Horssen P, et al. Very high intact-protein formula successfully provides protein intake according to nutritional recommendations in overweight critically ill patients: a double-blind randomized trial. Crit Care 2018;22:156.ArticlePubMedPMC
- 85. Azevedo JRA, Lima HCM, Montenegro WS, Souza SCC, Nogueira IROM, Silva MM, et al. Optimized calorie and high protein intake versus recommended caloric-protein intake in critically ill patients: a prospective, randomized, controlled phase II clinical trial. Rev Bras Ter Intensiva 2019;31:171-9. ArticlePubMedPMC
- 86. Chapple LS, Summers MJ, Bellomo R, Chapman MJ, Davies AR, Ferrie S, et al. TARGET Investigator Collaborative and the ANZICS Clinical Trials Group. Use of a high-protein enteral nutrition formula to increase protein delivery to critically ill patients: a randomized, blinded, parallel-group, feasibility trial. JPEN J Parenter Enteral Nutr 2021;45:699-709. ArticlePubMedPDF
- 87. Dresen E, Weißbrich C, Fimmers R, Putensen C, Stehle P. Medical high-protein nutrition therapy and loss of muscle mass in adult ICU patients: a randomized controlled trial. Clin Nutr 2021;40:1562-70. ArticlePubMed
- 88. Nakamura K, Nakano H, Naraba H, Mochizuki M, Takahashi Y, Sonoo T, et al. High protein versus medium protein delivery under equal total energy delivery in critical care: a randomized controlled trial. Clin Nutr 2021;40:796-803. ArticlePubMed
- 89. Heyland DK, Patel J, Compher C, Rice TW, Bear DE, Lee ZY, et al. EFFORT Protein Trial team. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023;401:568-76. ArticlePubMed
- 90. Lee ZY, Yap CSL, Hasan MS, Engkasan JP, Barakatun-Nisak MY, Day AG, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2021;25:260.ArticlePubMedPMCPDF
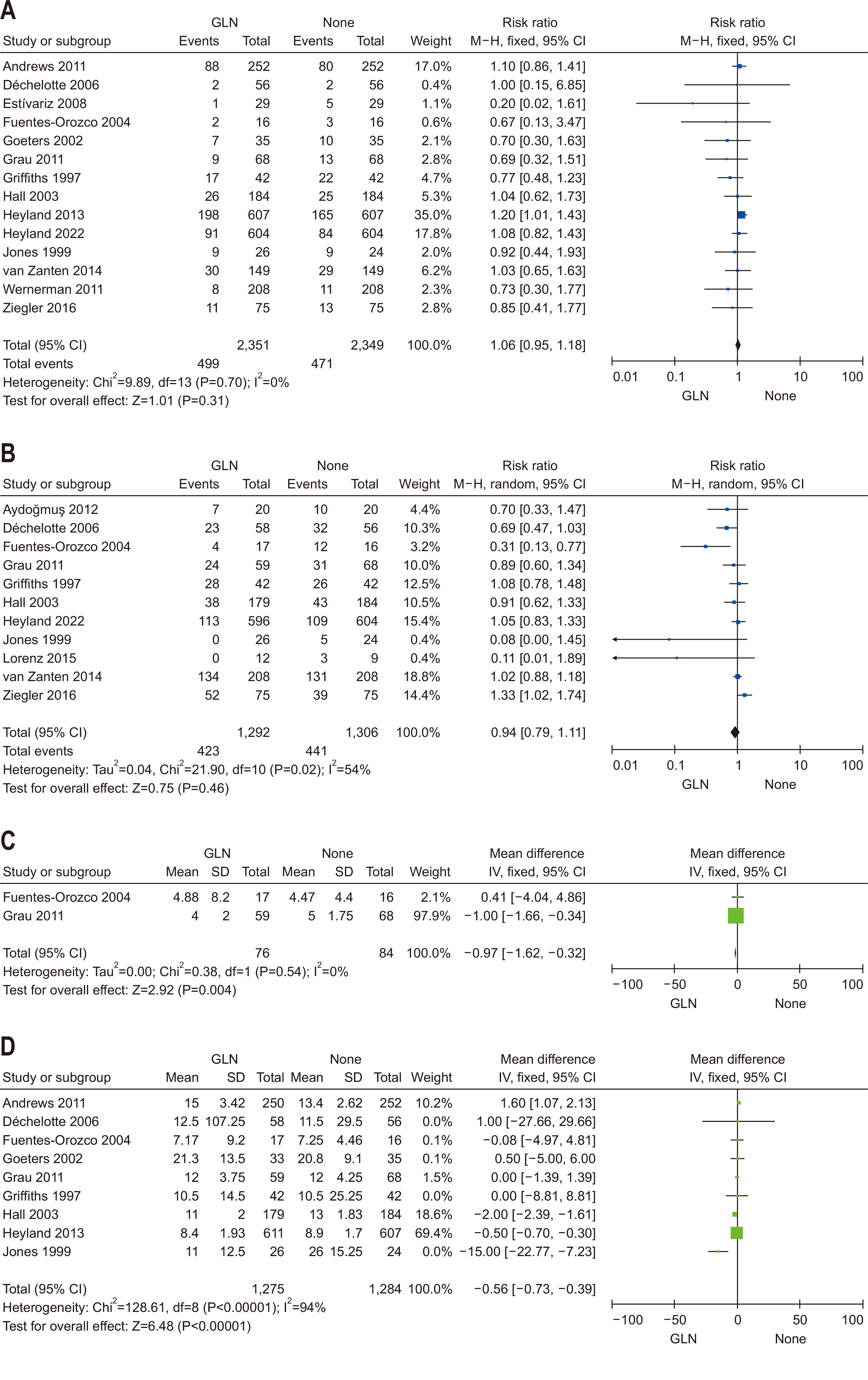
- 91. Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Scottish Intensive care Glutamine or seleNium Evaluative Trial Trials Group. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542. ArticlePubMed
- 92. Aydoğmuş MT, Tomak Y, Tekin M, Katı I, Hüseyinoğlu U. 2012;Glutamine supplemented parenteral nutrition to prevent ventilator-associated pneumonia in the intensive care unit. Balkan Med J 29:414-8. ArticlePubMedPMC
- 93. Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med 2006;34:598-604. ArticlePubMed
- 94. Estívariz CF, Griffith DP, Luo M, Szeszycki EE, Bazargan N, Dave N, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN J Parenter Enteral Nutr 2008;32:389-402. ArticlePubMedPMCPDF
- 95. Fuentes-Orozco C, Anaya-Prado R, González-Ojeda A, Arenas-Márquez H, Cabrera-Pivaral C, Cervantes-Guevara G, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin Nutr 2004;23:13-21. ArticlePubMed
- 96. Goeters C, Wenn A, Mertes N, Wempe C, Van Aken H, Stehle P, et al. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit Care Med 2002;30:2032-7. ArticlePubMed
- 97. Grau T, Bonet A, Miñambres E, Piñeiro L, Irles JA, Robles A, et al. Metabolism, Nutrition Working Group, SEMICYUC, Spain. The effect of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit Care Med 2011;39:1263-8. ArticlePubMed
- 98. Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997;13:295-302. ArticlePubMed
- 99. Hall JC, Dobb G, Hall J, de Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med 2003;29:1710-6. ArticlePubMedPDF
- 100. Heyland DK, Wibbenmeyer L, Pollack J, Friedman B, Turgeon AF, Eshraghi N, et al. RE-ENERGIZE Trial Team. A randomized trial of enteral glutamine for treatment of burn injuries. N Engl J Med 2022;387:1001-10. ArticlePubMed
- 101. Jones C, Palmer TE, Griffiths RD. Randomized clinical outcome study of critically ill patients given glutamine-supplemented enteral nutrition. Nutrition 1999;15:108-15. ArticlePubMed
- 102. Lorenz KJ, Schallert R, Daniel V. Immunonutrition - the influence of early postoperative glutamine supplementation in enteral/parenteral nutrition on immune response, wound healing and length of hospital stay in multiple trauma patients and patients after extensive surgery. GMS Interdiscip Plast Reconstr Surg DGPW 2015;4:Doc15.ArticlePubMedPMC
- 103. van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. 2014;High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. JAMA 312:514-24. ArticlePubMed
- 104. Wernerman J, Kirketeig T, Andersson B, Berthelson H, Ersson A, Friberg H, et al. Scandinavian Critical Care Trials Group. Scandinavian glutamine trial: a pragmatic multi-centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiol Scand 2011;55:812-8. ArticlePubMed
- 105. Ziegler TR, May AK, Hebbar G, Easley KA, Griffith DP, Dave N, et al. Efficacy and safety of glutamine-supplemented parenteral nutrition in surgical ICU patients: an american multicenter randomized controlled trial. Ann Surg 2016;263:646-55. ArticlePubMed
- 106. Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489-97. ArticlePubMed
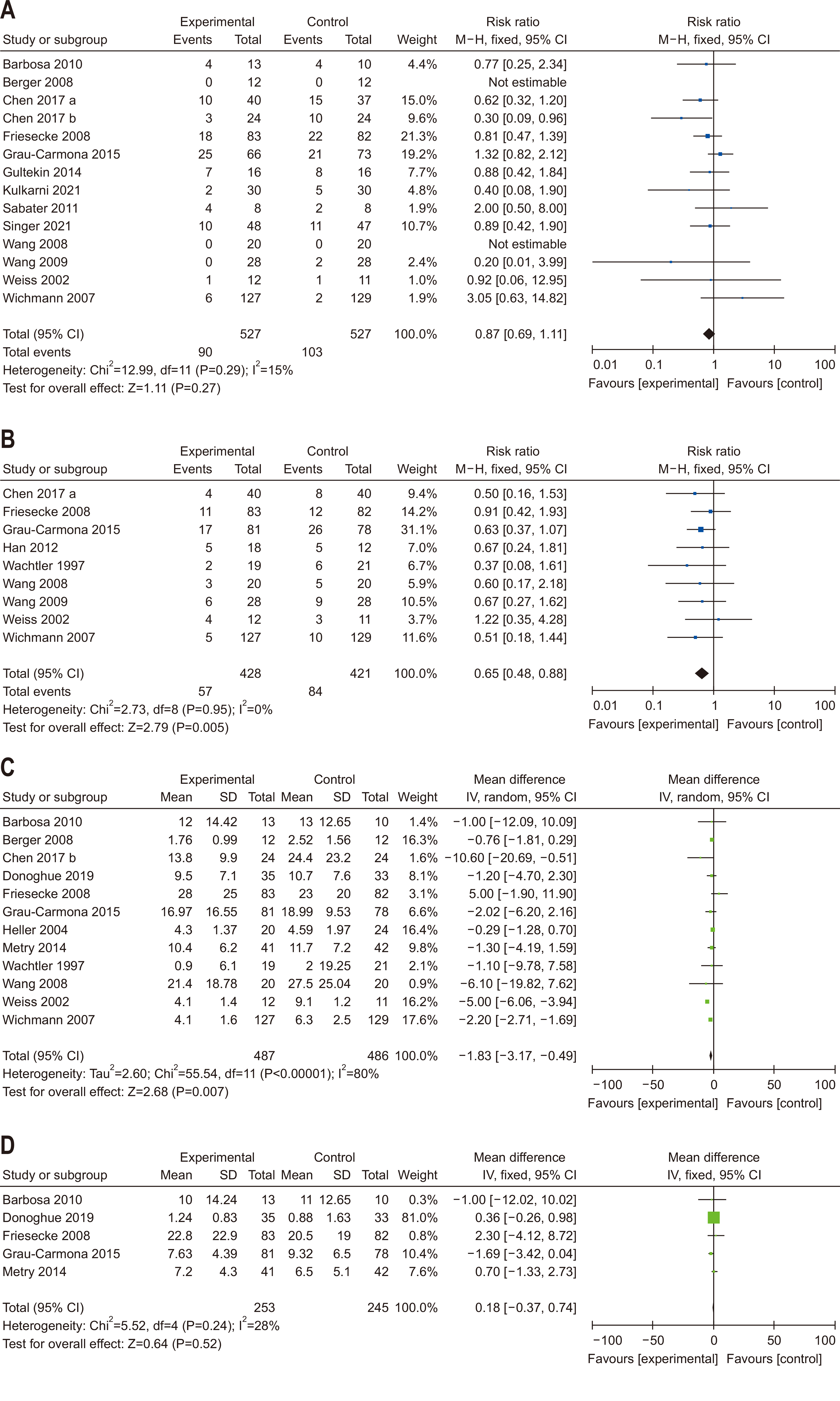
- 107. Wachtler P, König W, Senkal M, Kemen M, Köller M. Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma 1997;42:191-8. ArticlePubMed
- 108. Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr 2002;87 Suppl 1:S89-94. ArticlePubMed
- 109. Heller AR, Rössel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, et al. Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer 2004;111:611-6. ArticlePubMed
- 110. Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 2007;35:700-6. ArticlePubMed
- 111. Berger MM, Tappy L, Revelly JP, Koletzko BV, Gepert J, Corpataux JM, et al. Fish oil after abdominal aorta aneurysm surgery. Eur J Clin Nutr 2008;62:1116-22. ArticlePubMedPDF
- 112. Friesecke S, Lotze C, Köhler J, Heinrich A, Felix SB, Abel P. Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med 2008;34:1411-20. ArticlePubMedPDF
- 113. Wang X, Li W, Li N, Li J. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr 2008;32:236-41. ArticlePubMed
- 114. Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation 2009;32:304-9. ArticlePubMedPDF
- 115. Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care 2010;14:R5.ArticlePubMedPMCPDF
- 116. Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects of an omega-3 fatty acid-enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Nutr Metab (Lond) 2011;8:22.ArticlePubMedPMCPDF
- 117. Han YY, Lai SL, Ko WJ, Chou CH, Lai HS. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract 2012;27:91-8. ArticlePubMedPDF
- 118. Gultekin G, Sahin H, Inanc N, Uyanik F, Ok E. Impact of omega-3 and omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak J Med Sci 2014;30:299-304. ArticlePubMedPMC
- 119. Metry AA, Abdelaal W, Ragaei M, Refaat M, Nakhla G. SMOFlipid versus intralipid in postoperative ICU patients. Enliven: J Anesthesiol Crit Care Med 2014;1:015.Article
- 120. Grau-Carmona T, Bonet-Saris A, García-de-Lorenzo A, Sánchez-Alvarez C, Rodríguez-Pozo A, Acosta-Escribano J, et al. Influence of n-3 polyunsaturated fatty acids enriched lipid emulsions on nosocomial infections and clinical outcomes in critically ill patients: ICU lipids study. Crit Care Med 2015;43:31-9. ArticlePubMed
- 121. Chen H, Wang W, Hong C, Zhang M, Hong Y, Wang S, et al. Omega-3 fish oil reduces mortality due to severe sepsis with acute gastrointestinal injury grade iii. Pharmacogn Mag 2017;13:407-12. ArticlePubMedPMC
- 122. Chen H, Wang W, Hong Y, Zhang H, Hong C, Liu X. Single-blinded, randomized, and controlled clinical trial evaluating the effects of omega-3 fatty acids among septic patients with intestinal dysfunction: a pilot study. Exp Ther Med 2017;14:1505-11. ArticlePubMedPMC
- 123. Donoghue V, Schleicher GK, Spruyt MGL, Malan L, Nel DG, Calder PC, et al. Four-oil intravenous lipid emulsion effect on plasma fatty acid composition, inflammatory markers and clinical outcomes in acutely ill patients: a randomised control trial (Foil fact). Clin Nutr 2019;38:2583-91. ArticlePubMed
- 124. Kulkarni AV, Anand L, Vyas AK, Premkumar M, Choudhury AK, Trehanpati N, et al. Omega-3 fatty acid lipid emulsions are safe and effective in reducing endotoxemia and sepsis in acute-on-chronic liver failure: an open-label randomized controlled trial. J Gastroenterol Hepatol 2021;36:1953-61. ArticlePubMedPDF
- 125. Singer P, Bendavid I, Mesilati-Stahy R, Green P, Rigler M, Lev S, et al. Enteral and supplemental parenteral nutrition enriched with omega-3 polyunsaturated fatty acids in intensive care patients - a randomized, controlled, double-blind clinical trial. Clin Nutr 2021;40:2544-54. ArticlePubMed
- 126. Pradelli L, Mayer K, Klek S, Omar Alsaleh AJ, Clark RAC, Rosenthal MD, et al. ω-3 Fatty-acid enriched parenteral nutrition in hospitalized patients: systematic review with meta-analysis and trial sequential analysis. JPEN J Parenter Enteral Nutr 2020;44:44-57. ArticlePubMedPMCPDF
- 127. Pradelli L, Klek S, Mayer K, Omar Alsaleh AJ, Rosenthal MD, Heller AR, et al. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: systematic review with meta-analysis and cost-effectiveness analysis. Crit Care 2020;24:634.ArticlePubMedPMCPDF
References
Figure & Data
REFERENCES
Citations

- Enteral Nutrition Versus a Combination of Enteral and Parenteral Nutrition in Critically Ill Adult Patients in the Intensive Care Unit: An Overview of Systematic Reviews and Meta-Analysis
Paraskevi Papanikolaou, Xenophon Theodoridis, Androniki Papaemmanouil, Niki N. Papageorgiou, Alexandra Tsankof, Anna-Bettina Haidich, Christos Savopoulos, Konstantinos Tziomalos
Journal of Clinical Medicine.2025; 14(3): 991. CrossRef
- Figure
- Related articles
-
- A practical guide for enteral nutrition from the Korean Society for Parenteral and Enteral Nutrition: Part II. selection and initiation of enteral feeding routes
- A practical guide for enteral nutrition from the Korean Society for Parenteral and Enteral Nutrition: Part I. prescribing enteral nutrition orders
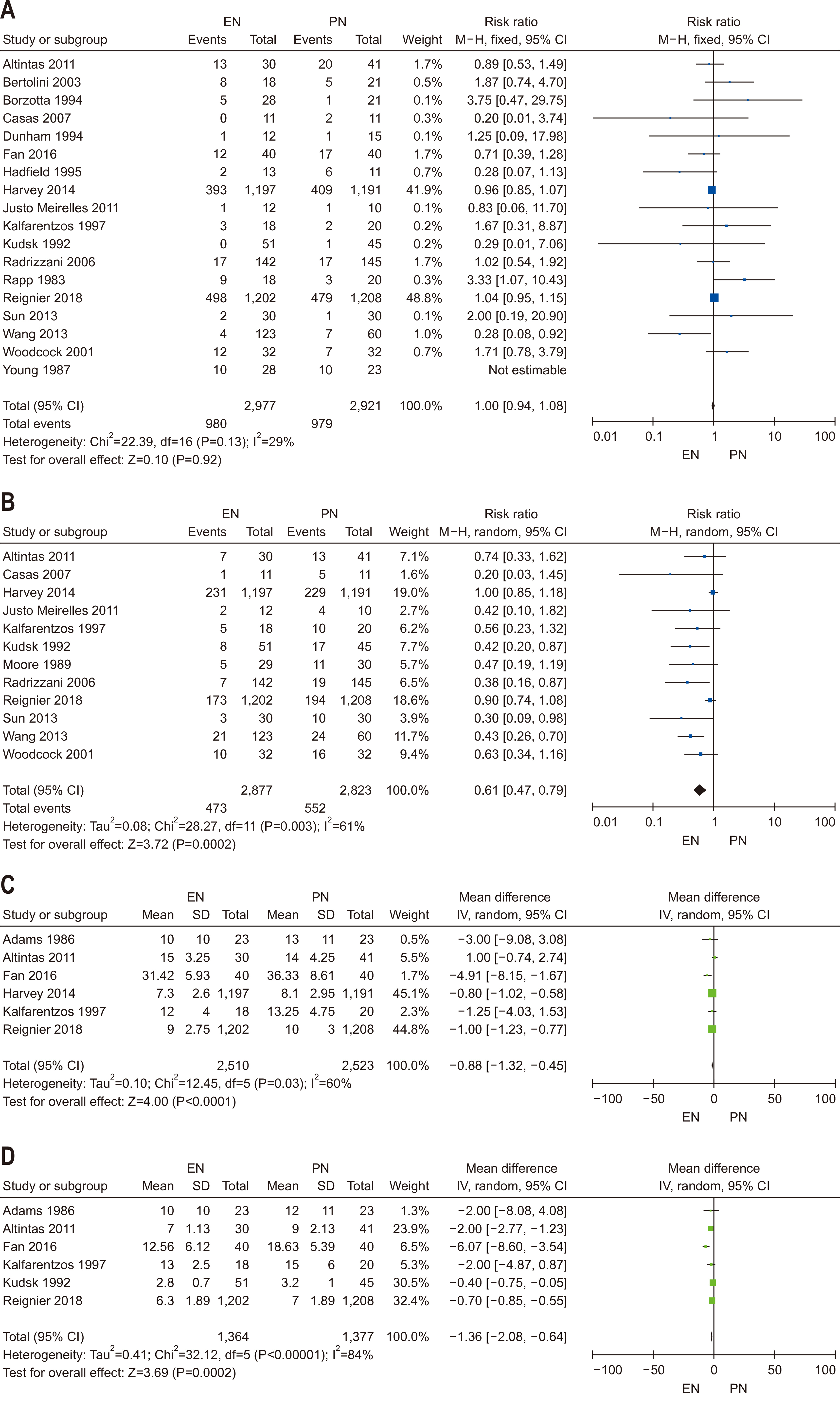
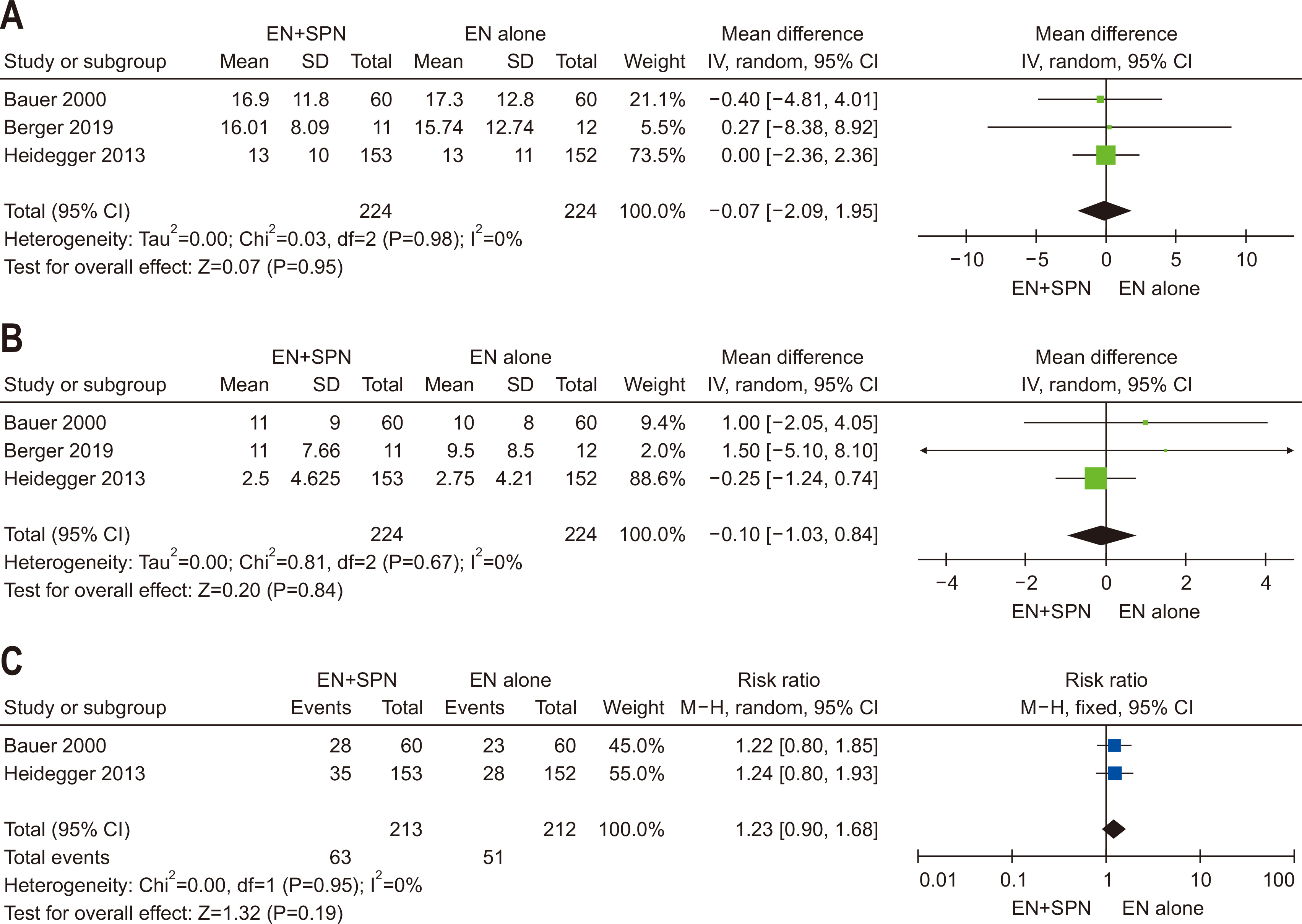
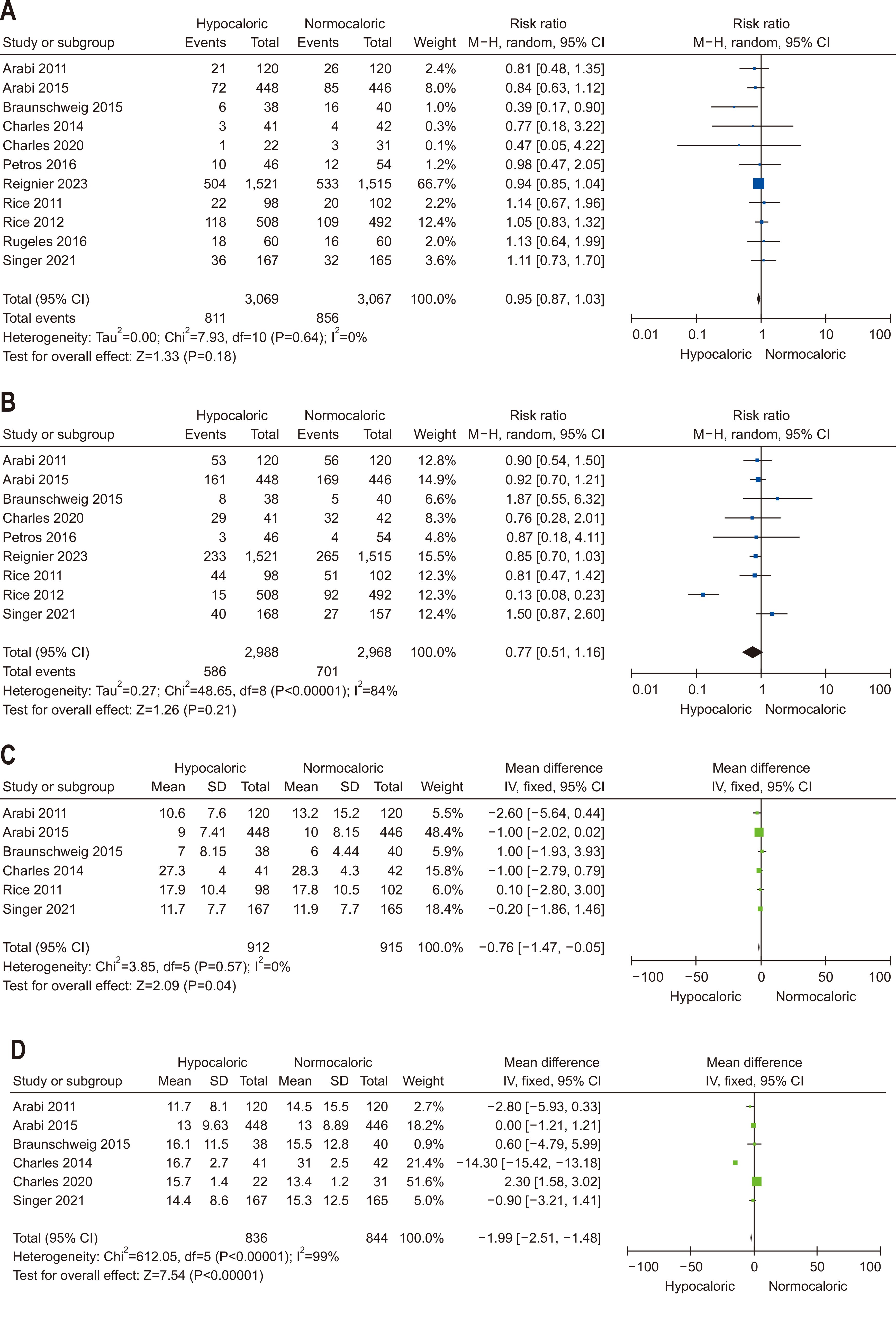
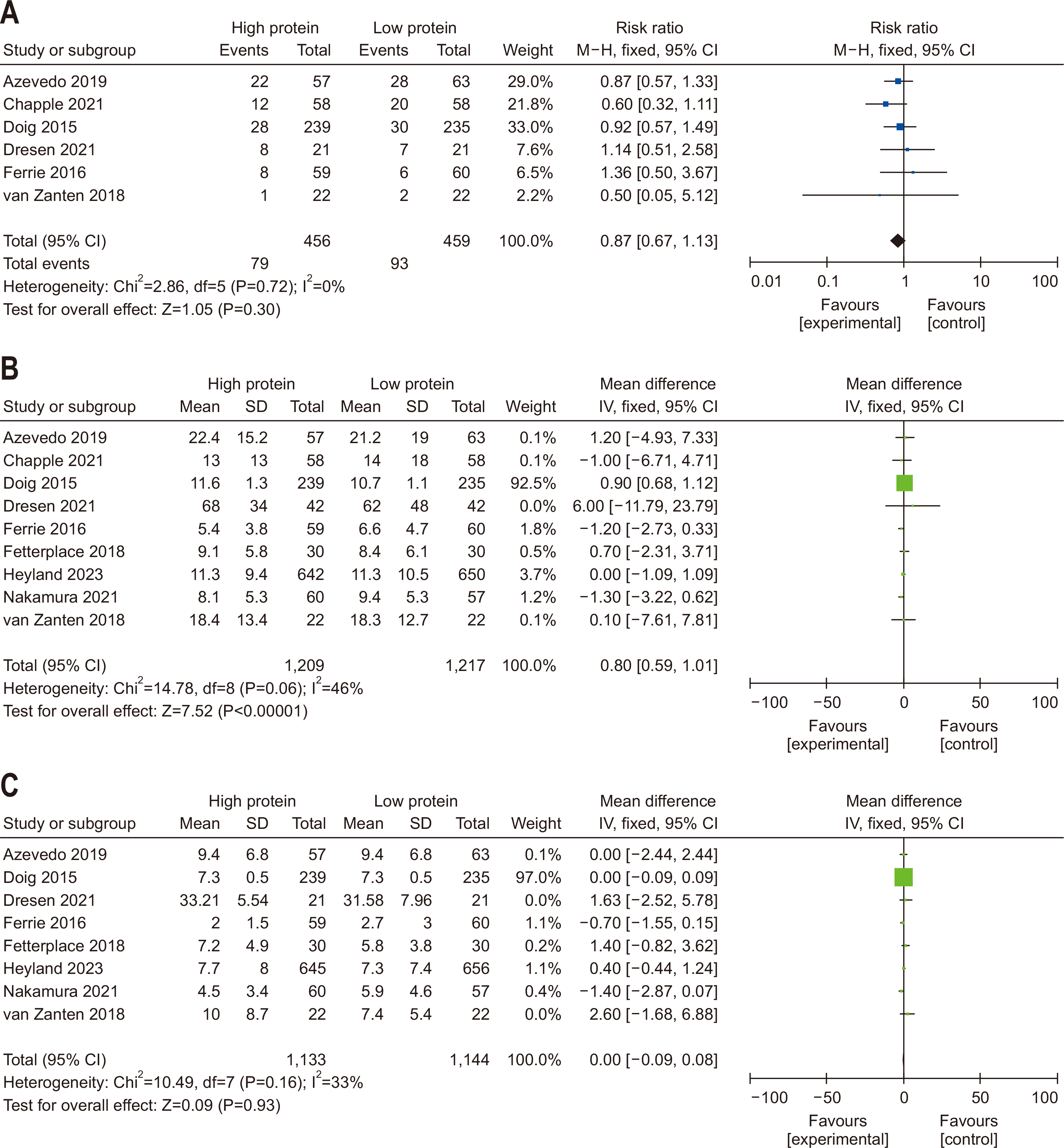
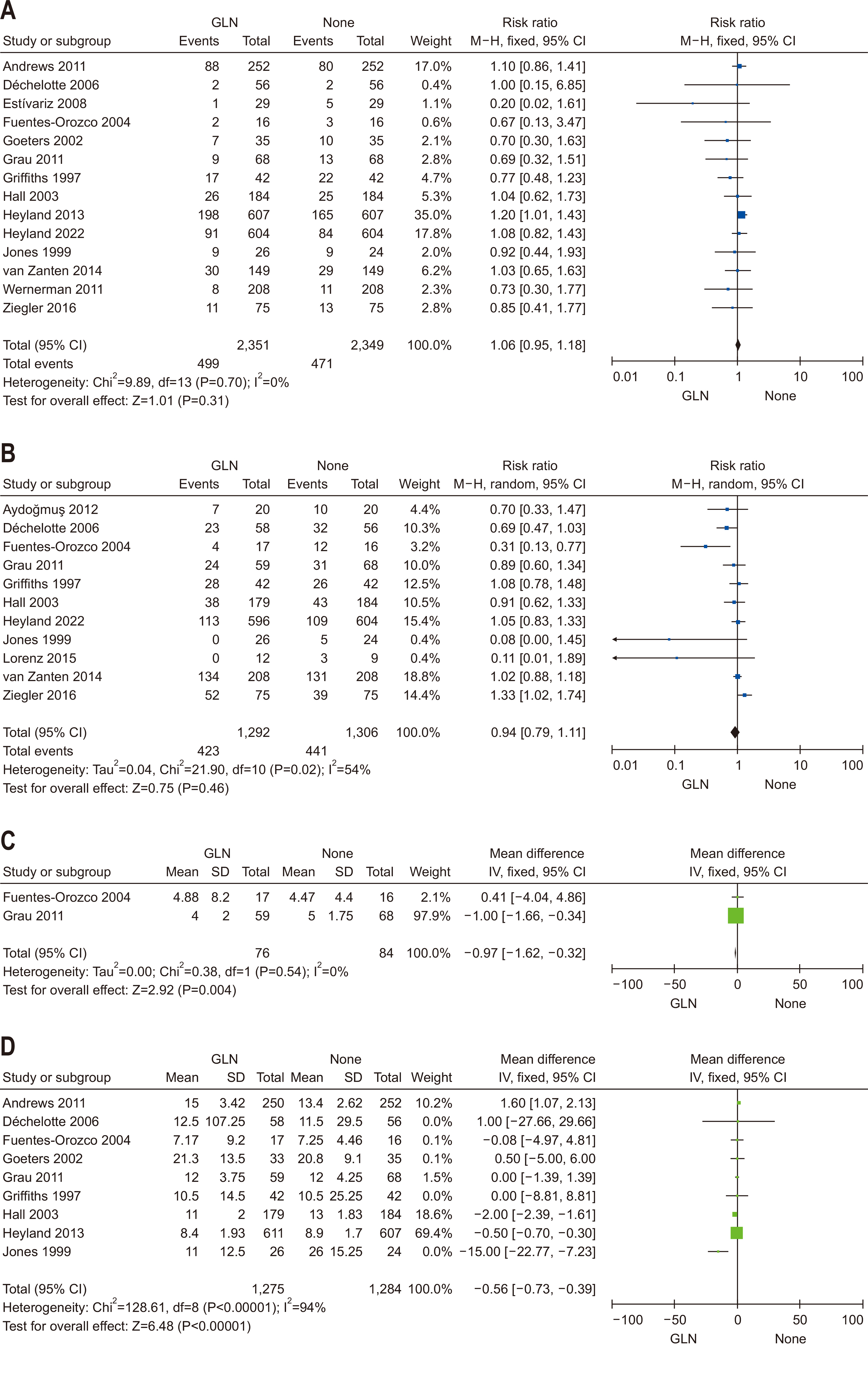
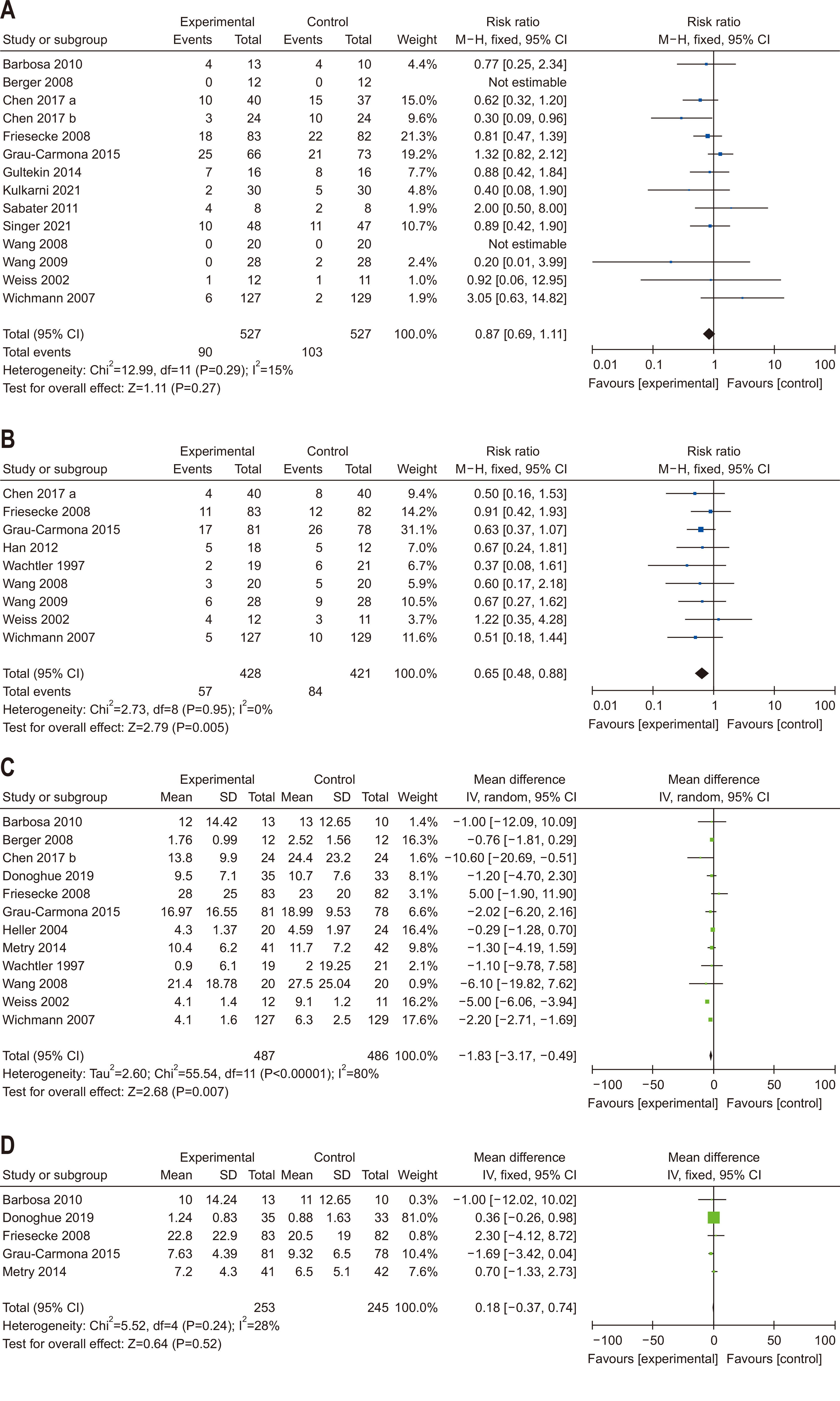
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Search terms for literature search
| Question | Terms |
|---|---|
| Q1 | “Enteral nutrition”[Mesh] , “Critical Illness”[Mesh] |
| Q2 | (“Critical Illness”[MH]) OR (“Sepsis”[MH]) OR (“Shock, Septic”[MH]) OR (“Critical illnesses”[ALL] or “Critically ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL] or “sepsis”[ALL] or “septic shock”[ALL]), (“Enteral Nutrition”[MH]) or (“Enteral nutrition”[ALL] or “Enteral Feeding”[ALL] or “tube Feeding”[ALL]), (“Parenteral Nutrition”[MH]) or (“Parenteral nutrition”[ALL] or “Parenteral Feeding”[ALL] or “Intravenous Feeding”[ALL]) |
| Q3 | (“Critical Illness”[MH]) OR (“Critical Illnesses”[ALL] or “Critically Ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL]), (“Enteral Nutrition”[MH]) or (“Enteral nutrition”[ALL] or “Enteral Feeding”[ALL] or “Force Feeding”[ALL] or “early enteral nutrition”[ALL]), (“Parenteral Nutrition”[MH]) or (“Parenteral nutrition”[ALL] or “Parenteral Feeding”[ALL] or “Intravenous Feeding”[ALL] or “supplemental parenteral nutrition”[ALL]) |
| Q4 | (“Critical Illness”[MH]) OR (“Critical Illnesses”[ALL] or “Critically Ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL]) (“Caloric Restriction”[MH]) or (“Caloric Restrict”[ALL] or “Calorie Restrict”[ALL] or “energy restrict”[ALL] or “Low-Calorie Diet”[ALL]), (“normocaloric feeding”[ALL]) |
| Q5 | (“Critical Illness”[MH]) OR (“Critical Illnesses”[ALL] or “Critically Ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL]), (“Diet, High-Protein”[MH]) OR (“Diet, High Protein”[ALL] or “High-Protein Diet”[ALL] or “high amino acid”[ALL]), (“Diet, Protein-Restricted”[MH]) OR (“Diet, Protein-Restricted”[ALL] or “Diet, Low-Protein”[ALL] or “Diet*, Protein-Free”[ALL]) |
| Q6 | (“Critical Illness”[MH]) OR (“Critical Illnesses”[ALL] or “Critically Ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL]), (“Enteral Nutrition”[MH]) OR (“Parenteral Nutrition”[MH]) OR (“Enteral nutrition”[ALL] or “Enteral Feeding”[ALL] or “Force Feeding”[ALL] or “Parenteral nutrition”[ALL] or “Parenteral Feeding”[ALL] or “Intravenous Feeding”[ALL]), (“glutamine”[ALL]) |
| Q7 | (“Critical Illness”[MH]) OR (“Sepsis”[MH]) OR (“Shock, Septic”[MH]) OR (“Critical illnesses”[ALL] or “Critically ill”[ALL] or “ICU”[ALL] or “intensive care”[ALL] or “sepsis”[ALL] or “septic shock”[ALL]), (“Fatty Acids, Omega-3”[MH]) OR (“Omega-3 Fatty Acid”[ALL] or “n-3 Oil”[ALL]), (“Fish Oils”[MH]) OR (“Fish Oil”[ALL] or “Oil Fish”[ALL]), (“Fat Emulsions, Intravenous”[MH]) OR (“Intravenous Lipid Emulsion”ALL] or “Fat Emulsions, Intravenous”[ALL] or “Intravenous Fat Emulsions”[ALL]) |
Quality of evidence grade
| Level | Definition |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate, but there is a possibility that it is substantially different |
| Low | Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate |
| Very low | We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate |
Grade of recommendation
| Grade | Definition |
|---|---|
| Strong recommendation | Given the balance of benefits and harms, evidence level, values and preferences, and resource considerations, this treatment is strongly recommended in most clinical situations |
| Conditional recommendation | The use of this treatment may vary depending on the clinical situation or patient/societal values. It is suggested that the treatment be used selectively or conditionally |
| Conditional recommendation against | The harms of this treatment may outweigh the benefits, and considering the clinical context or patient/societal values, it is advised not to use the treatment in certain situations or under specific conditions |
| Strong recommendation against | The harms of this treatment clearly outweigh the benefits, and considering the clinical context or patient/societal values, it is advised not to use the treatment in most clinical situations |
| Conditional recommendation both (inconclusive) | The evidence is insufficient to make a clear recommendation either for or against the treatment. Given the variability in evidence, clinical context, patient preferences, and values, the decision to use the treatment should be made on a case-by-case basis, considering both the potential benefits and harms in the specific clinical situation |
| Expert consensus recommendation | Although there is limited clinical evidence, based on clinical experience and expert consensus, the use of this treatment is recommended considering its benefits and harms, evidence level, values and preferences, and resource availability |
Consensus strength
| Consensus strength | % agreement |
|---|---|
| Strong consensus | >90 |
| Consensus | >75–90 |
| Majority consensus | >50–75 |
| No consensus | <50 |
Summary for recommendations
| Guideline question | Grade recommendation | Grade of recommendation | Quality of evidence | Consensus |
|---|---|---|---|---|
| Question 1. In critically ill adult patients, does the provision of early EN compared with delayed EN affect clinical outcomes? | In critically ill adult patients, early enteral nutrition is recommended if hemodynamic stability is achieved within 48 hours of the start of treatment | Conditional recommendation | Low | Strong (90%) |
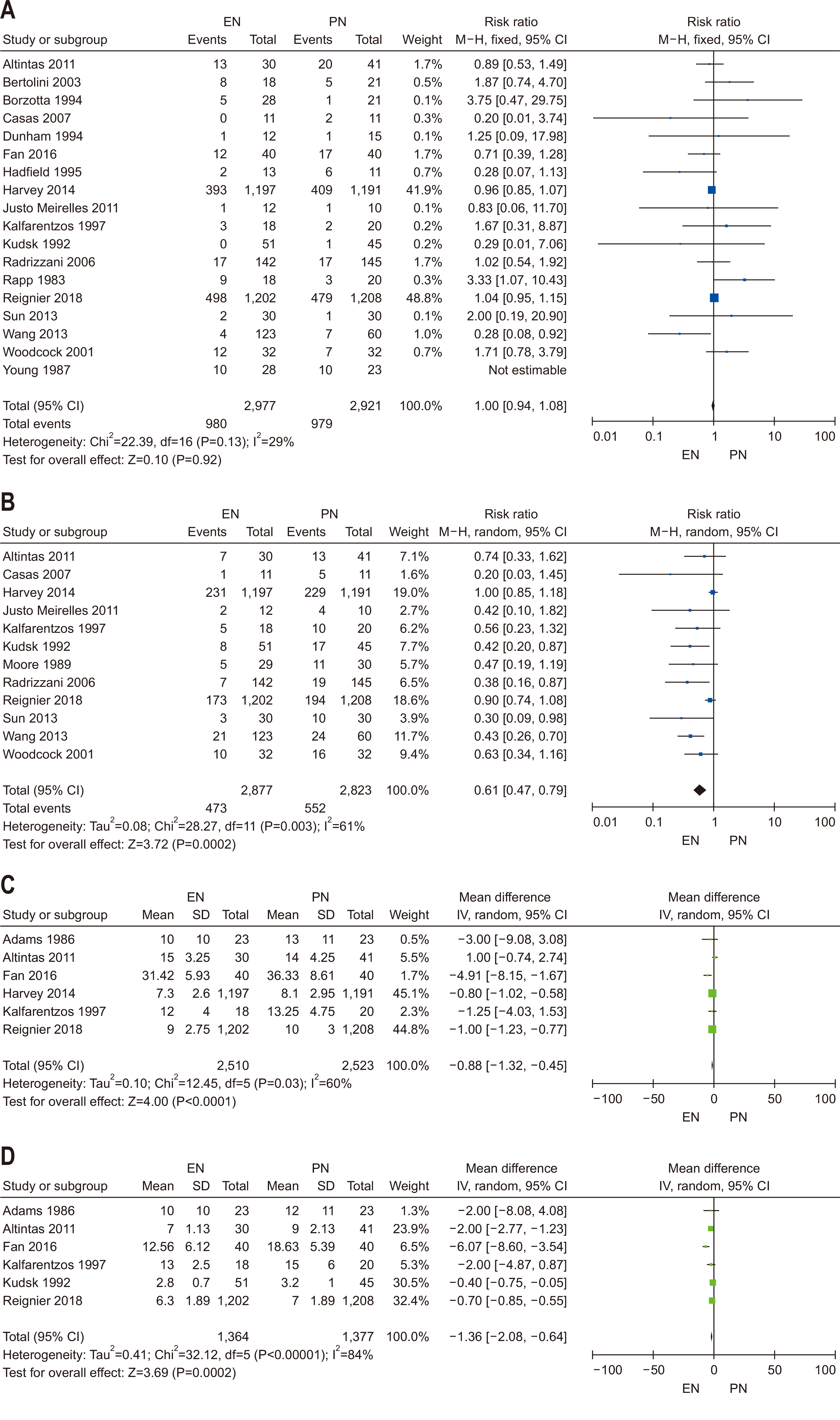
| Question 2. In critically ill adult patients, does early EN compared with early PN result in superior clinical outcomes? | In critically ill adult patients who are unable to take oral intake, early enteral nutrition should be prioritized over early parenteral nutrition | Strong recommendation | Moderate | Strong (93%) |
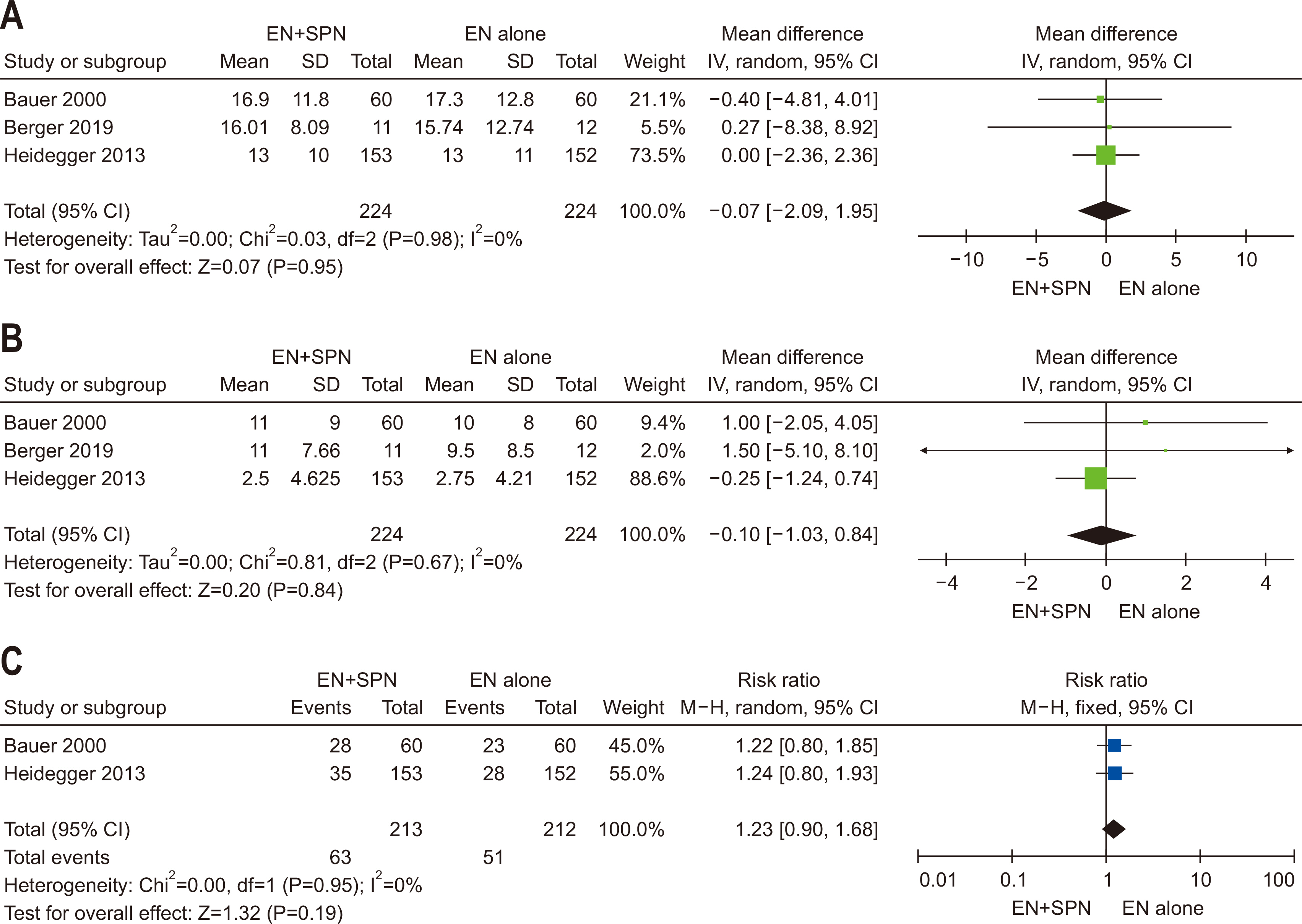
| Question 3. In critically ill adult patients receiving early EN, does the provision of SPN to meet energy targets, compared with no SPN during the first week of critical illness, affect clinical outcomes? | Based on studies showing no significant clinical benefits of providing SPN early in the ICU stay, SPN initiation within the first seven days of ICU admission is not recommended | Conditional recommendation against | Low | Majority agreement (74%) |
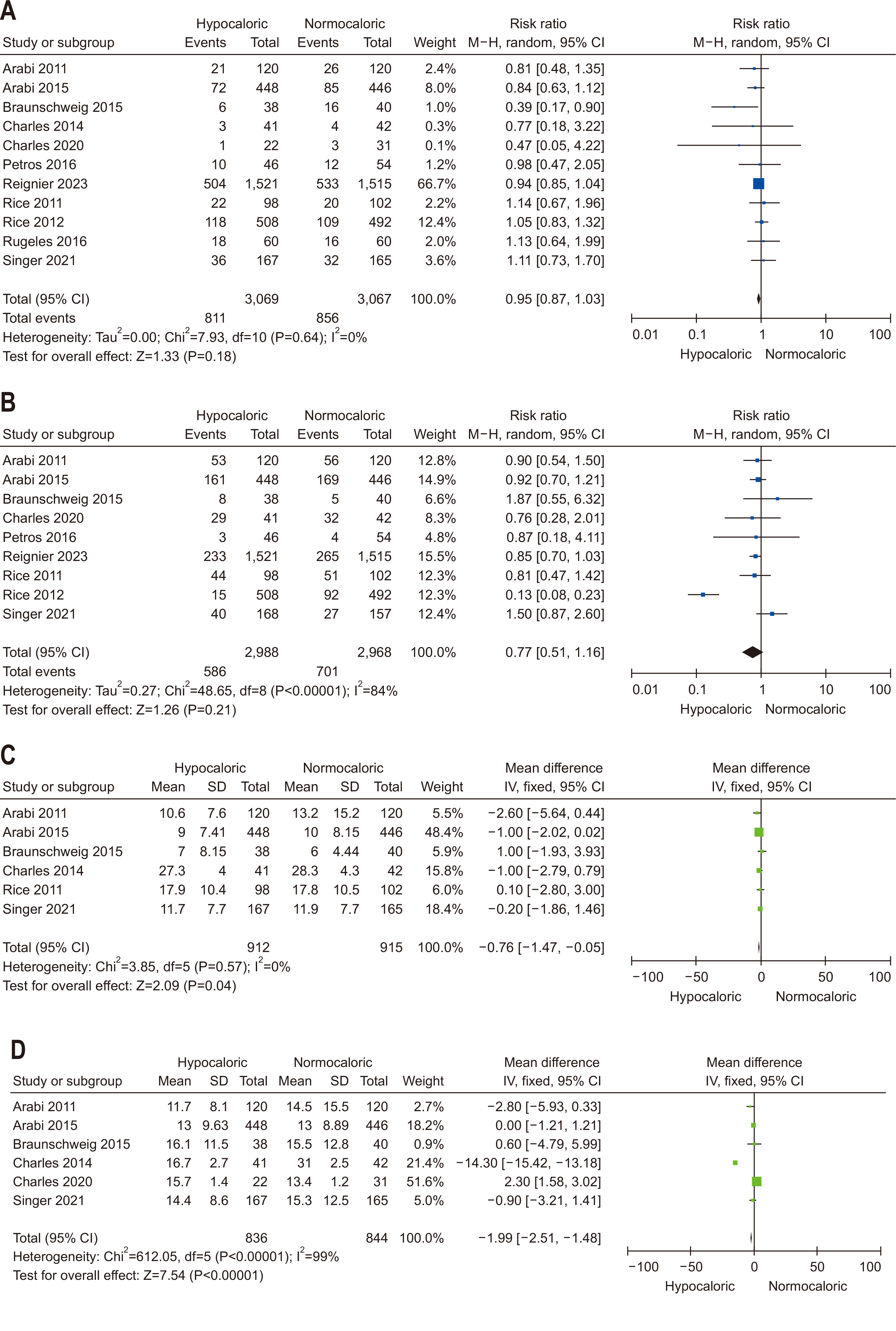
| Question 4. In critically ill adult patients, does provision early vs. delayed EN affect clinical outcomes? | During the initial seven days of ICU admission, restricted calorie supply (less than 70% of the predicted energy requirement) is recommended | Conditional recommendation | Moderate | Strong (93%) |
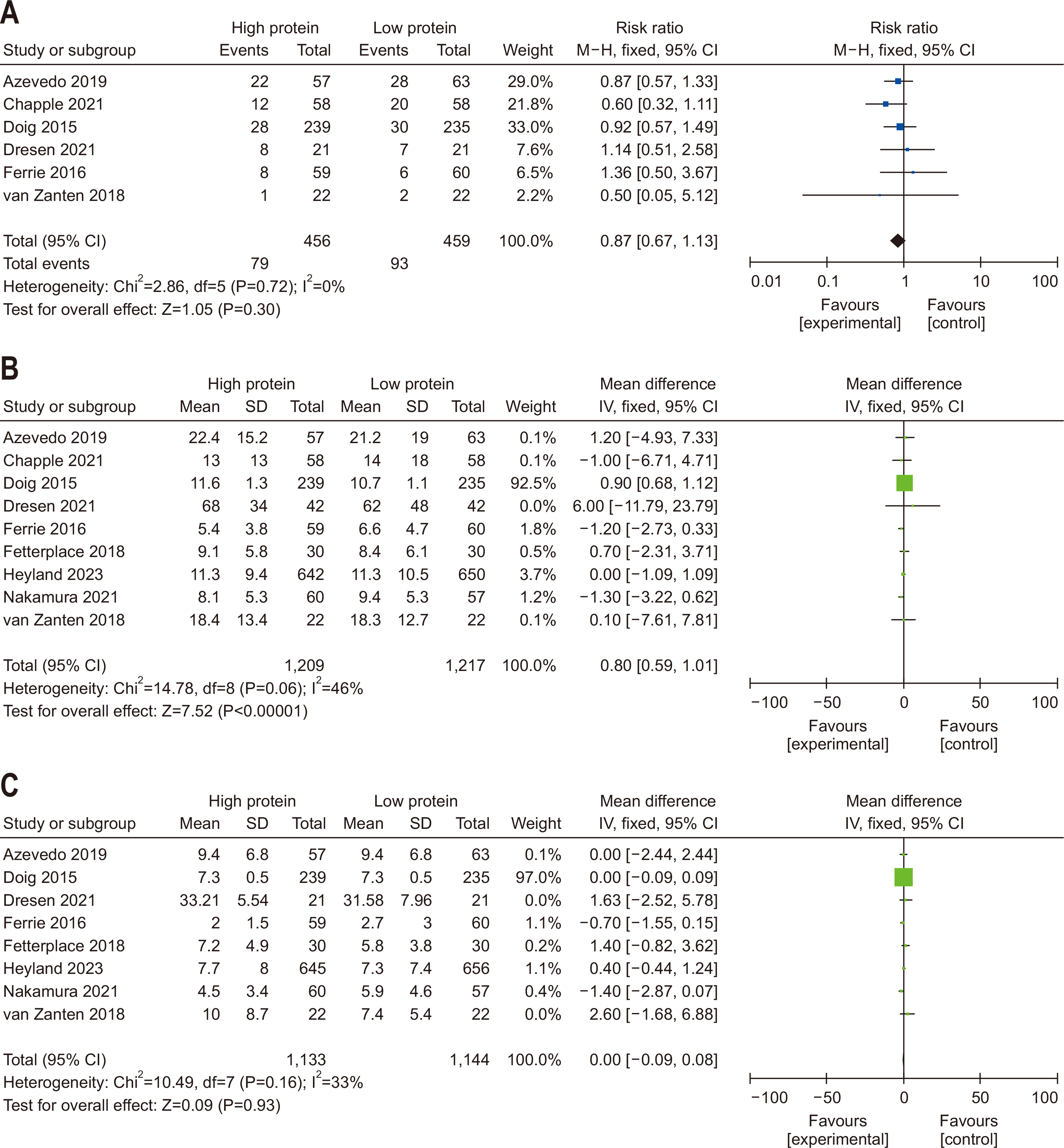
| Question 5. In critically ill adult patients, does high protein intake compared with low protein intake improve clinical outcomes? | In critically ill adult patients, a gradual increase in protein intake to 1.2–1.3 g/kg/d is recommended | Conditional recommendation | Moderate | Strong (97%) |
| Question 6. In critically ill adult patients, does provision of enteral or parenteral glutamine impact clinical outcomes? | In critically ill adult patients, additional enteral or parenteral glutamine supplementation is not recommended | Conditional recommendation against | Low | Strong (90%) |
| Question 7. In critically ill adult patients, does provision of high FO containing ILEs compared with low FO-containing ILEs affect clinical outcomes? | In critically ill adult patients, intravenous lipid emulsions containing fish oil may be beneficial for clinical outcomes and should be considered for administration | Conditional recommdendation | Low | Consensus (81%) |
EN = enteral nutrition; PN = parenteral nutrition; SPN = supplemental PN; FO = fish oil; ILEs = intravenous lipid emulsions; ICU = intensive care unit.
Summary of clinical outcomes related to early enteral nutrition vs. late enteral nutrition
| Outcomes | Authors | Study year | Findings | Reference |
|---|---|---|---|---|
| Mortality | Heyland et al. | 2003 | RR=0.52, 95% CI=0.25 to 1.08, P=0.08 | [ |
| Taylor et al. | 2016 | RR=0.70, 95% CI=0.49 to 1.00, P=0.05 | [ |
|
| Reintam Blaser et al. | 2017 | RR=0.76, 95% CI=0.52 to 1.11, P=0.149 | [ |
|
| Pu et al. | 2018 | OR=0.36, 95% CI=0.18 to 0.72, P=0.003 | [ |
|
| Kim et al. | 2021 | Mortality hazard ratio=0.413, 95% CI=0.174 to 0.984, P=0.046 | [ |
|
| Park et al. | 2016 | EN≤1.5 days 11.8% vs. EN>1.5 days 42.9%, P=0.018 | [ |
|
| Choi et al. | 2023 | EEN=14.1% vs. DEN 33.1%, P<0.001 | [ |
|
| Infections | Heyland et al. | 2003 | RR=0.66, 95% CI=0.36 to 1.22, P=0.19 | [ |
| Taylor et al. | 2016 | RR=0.74, 95% CI=0.58 to 0.93, P=0.01 | [ |
|
| Reintam Blaser et al. | 2017 | RR=0.64, 95% CI=0.46 to 0.90, P=0.010 | [ |
|
| Pu et al. | 2018 | OR=0.23, 95% CI=0.11 to 0.48, P<0.0001 | [ |
|
| Singer et al. | 2023 | RR=0.76, 95% CI=0.59 to 0.97, P<0.03 | [ |
|
| Length of stay | Marik and Zaloga | 2001 | MD=2.2 days, 95% CI=0.81 to 3.63 days, P=0.001 | [ |
| Pu et al. | 2018 | −15.31 days, 95% CI=−20.43 to −10.20, P<0.00001 | [ |
RR = risk ratio; CI = confidential interval; OR = odds ratio; EN = enteral nutrition; EEN = early enteral nutrition; DEN = delayed enteral nutrition; MD = mean difference.
EN = enteral nutrition; PN = parenteral nutrition; SPN = supplemental PN; FO = fish oil; ILEs = intravenous lipid emulsions; ICU = intensive care unit.
RR = risk ratio; CI = confidential interval; OR = odds ratio; EN = enteral nutrition; EEN = early enteral nutrition; DEN = delayed enteral nutrition; MD = mean difference.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite

