Scopus, KCI, KoreaMed

Search
- Page Path
- HOME > Search
- Impact of tube feeding after pancreaticoduodenectomy on nutritional intake and status: a retrospective cohort study in Japan
- Masaharu Ishida, Masahiro Iseki, Shuichiro Hayashi, Aya Noguchi, Hideaki Sato, Shingo Yoshimachi, Akiko Kusaka, Mitsuhiro Shimura, Shuichi Aoki, Daisuke Douchi, Takayuki Miura, Shimpei Maeda, Masamichi Mizuma, Kei Nakagawa, Takashi Kamei, Michiaki Unno
- Ann Clin Nutr Metab 2025;17(3):203-209. Published online December 1, 2025
- DOI: https://doi.org/10.15747/ACNM.25.0020
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Purpose
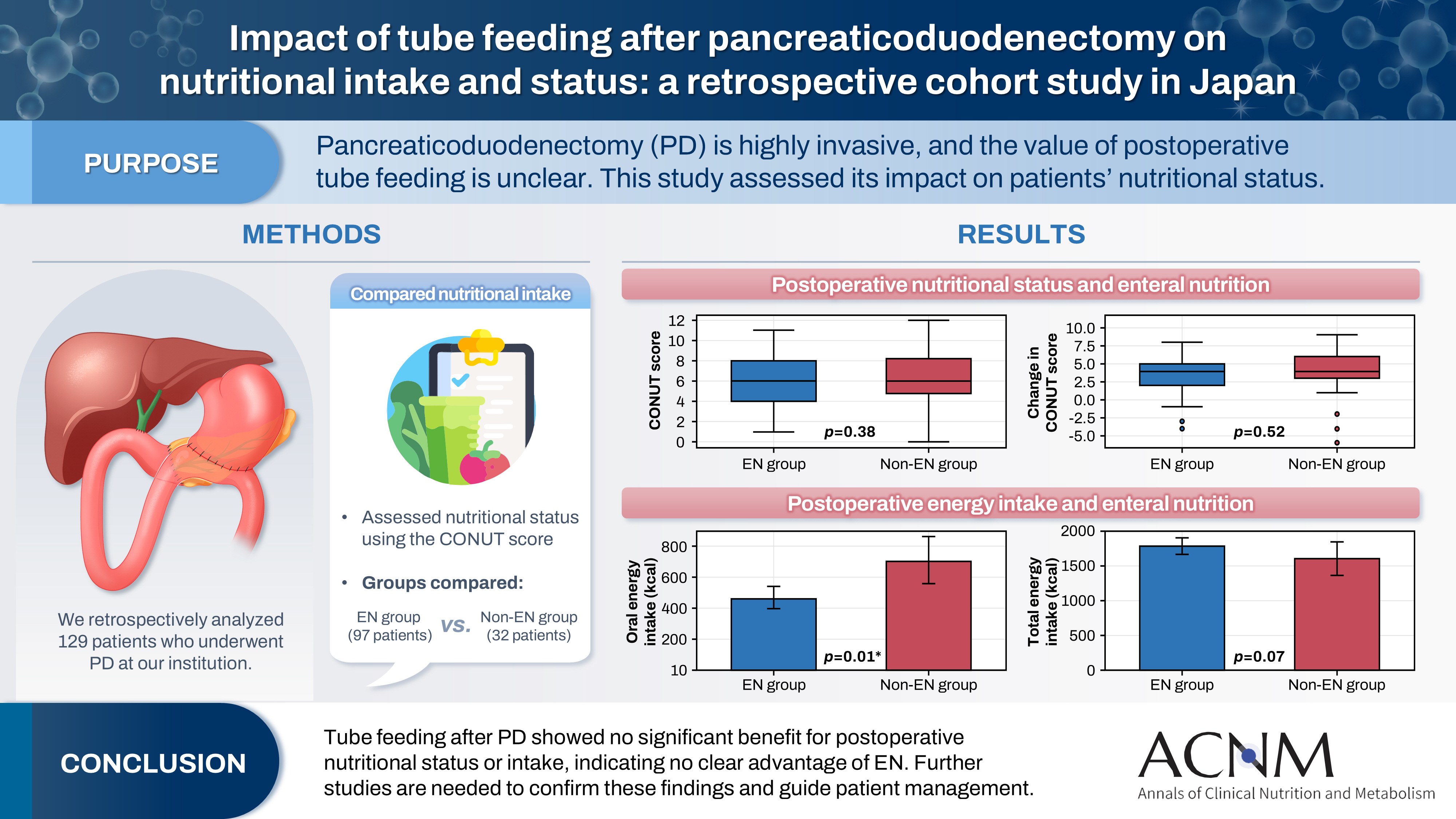
Pancreaticoduodenectomy (PD) is one of the most invasive procedures in gastrointestinal surgery. However, the clinical significance of postoperative tube feeding remains unclear. This study investigated the impact of enteral nutrition (EN) on the postoperative nutritional status of patients undergoing PD.
Methods
We retrospectively analyzed 129 patients who underwent PD at Tohoku University Hospital. Nutritional intake and status, evaluated using the Controlling Nutritional Status score, were compared between two groups: an EN group (97 patients) and a non-EN group (32 patients).
Results
There were no significant differences between the two groups in age, sex, body mass index, underlying diseases, operative duration, blood loss, postoperative pancreatic fistula, postoperative complications, delayed gastric emptying, or length of hospital stay. Although the EN group showed improvements in nutritional status both at discharge and compared with preoperative values, none of these changes reached statistical significance. Oral caloric intake was significantly higher in the non-EN group (P=0.01). In contrast, total energy intake was higher in the EN group, but this difference did not reach statistical significance (P=0.07).
Conclusion
Tube feeding after PD did not significantly influence postoperative nutritional status or overall nutritional intake. These findings suggest that EN offers no clear advantage over other approaches; however, further research is warranted to validate these results, refine existing guidelines, and optimize postoperative patient management.
- 646 View
- 12 Download

- Successful introduction of ERAS in pancreaticoduodenectomy: what is real minimally invasive surgery?
- Toshimi Kaido, Yosuke Miyachi, Koichiro Mitsuoka, Mariko Sambommatsu
- Ann Clin Nutr Metab 2025;17(2):156-161. Published online August 1, 2025
- DOI: https://doi.org/10.15747/ACNM.25.0014
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Purpose
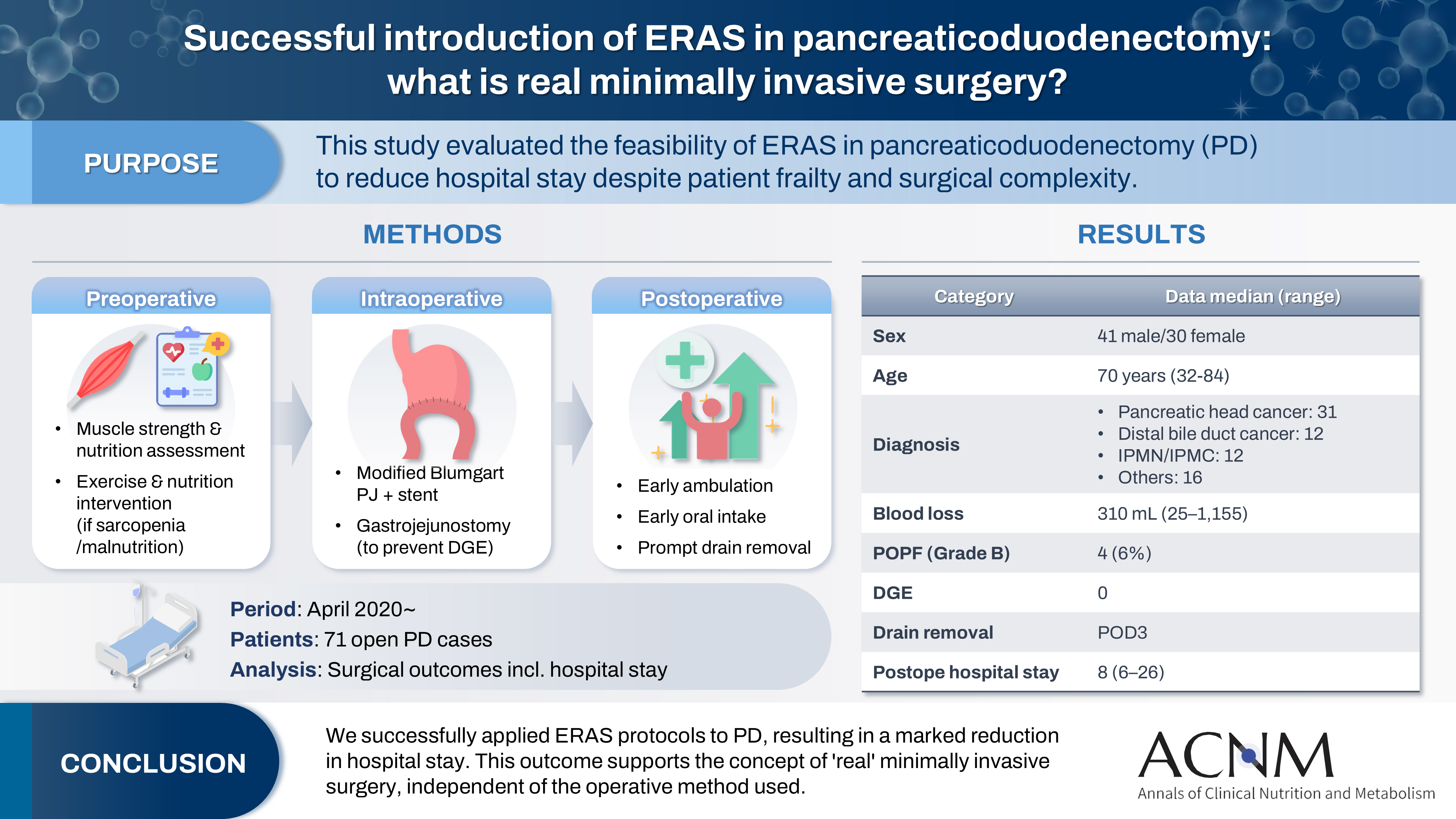
The introduction of Enhanced Recovery After Surgery (ERAS) protocols for pancreaticoduodenectomy (PD) has been considered challenging due to factors such as preexisting malnutrition, sarcopenia, the complexity of the surgery, and the high incidence of postoperative complications, including postoperative pancreatic fistula (POPF) and delayed gastric emptying (DGE). This study aimed to determine whether ERAS could be implemented in PD to achieve shorter postoperative hospital stays.
Methods
Our novel approach consists of three components. Preoperatively, we routinely assess patients' muscle strength and nutritional status and initiate exercise and nutritional interventions for those identified with sarcopenia or malnutrition. Intraoperatively, we perform pancreaticojejunostomy using a modified Blumgart’s technique with our stent placement policy and utilize new gastrojejunostomy methods to prevent DGE. Principles of postoperative management are early ambulation, early oral intake, and early drain removal. Since April 2020, we have employed this strategy and retrospectively evaluated its effectiveness. We enrolled 71 consecutive patients who underwent open PD with curative intent. Various surgical outcomes, including postoperative hospital stay, were analyzed.
Results
There were 41 men and 30 women, with a median age of 70 years. Preoperative diagnoses included pancreatic head cancer in 31, distal bile duct cancer in 12, and others. Median intraoperative blood loss was 310 mL. Grade B POPF occurred in four patients (6%). No cases of DGE were observed. The median postoperative hospital stay was 8 days (range, 6–26 days).
Conclusion
We successfully implemented ERAS protocols in PD and achieved a significantly reduced postoperative hospital stay. We propose that this approach is “real minimally invasive surgery," regardless of the surgical technique used.
- 3,966 View
- 19 Download

- Incidence and risk factors of nonalcoholic fatty liver disease after pancreaticoduodenectomy in Korea: a multicenter retrospective cohort study
- Chang-Sup Lim, Hongbeom Kim, In Woong Han, Won-Gun Yun, Eunchae Go, Jaewon Lee, Kyung Chul Yoon, So Jeong Yoon, Sang Hyun Shin, Jin Seok Heo, Yong Chan Shin, Woohyun Jung
- Ann Clin Nutr Metab 2024;16(3):125-133. Published online December 1, 2024
- DOI: https://doi.org/10.15747/ACNM.2024.16.3.125
-
 Abstract
Abstract
 PDF
PDF - Purpose: This study aimed to investigate the incidence, risk factors, and clinical course of nonalcoholic fatty liver disease (NAFLD) following pancreaticoduodenectomy, focusing on the role of adjuvant chemotherapy and other metabolic changes.
Methods: A retrospective analysis was conducted on 189 patients who underwent pancreaticoduodenectomy between 2013 and 2016. NAFLD was diagnosed using computed tomography (CT) imaging, defined as a liver-to-spleen attenuation ratio <0.9. Sarcopenia and sarcopenic obesity were assessed using preoperative CT scans. Logistic regression analysis was performed to identify risk factors for NAFLD development.
Results: The cumulative incidence of NAFLD increased over time, with rates of 15.9% at one year, 20.4% at three years, and 35.2% at five years post-pancreaticoduodenectomy. Adjuvant chemotherapy was identified as the only significant independent predictor of NAFLD development (odds ratio, 2.74; 95% confidence interval, 1.16-6.70; P=0.023). No significant associations were found between NAFLD and pancreatic enzyme replacement therapy (PERT), sarcopenia, or sarcopenic obesity. Serial analysis of NAFLD status in long-term survivors revealed dynamic changes, with some patients experiencing spontaneous remission or recurrence.
Conclusion: NAFLD is a common, progressive complication following pancreaticoduodenectomy, particularly in patients receiving adjuvant chemotherapy. Although no significant associations with PERT or sarcopenia were observed, these areas warrant further investigation. Long-term monitoring and targeted management strategies are recommended to address NAFLD in this population. Future prospective studies are needed to elucidate the natural history and contributing factors of NAFLD after pancreaticoduodenectomy.
- 3,662 View
- 61 Download

- Determination of the Stress Factor Calculated from the Changes in the Measured Resting Energy Expenditure with Indirect Calorimetry in Patients Undergoing Pancreaticoduodenectomy
- Seon Hyeong Kim, Baik Hwan Cho, Sook Bae Kim, Mi Jin Jeong, Hee Chul Yu
- J Clin Nutr 2017;9(2):62-67. Published online December 31, 2017
- DOI: https://doi.org/10.15747/jcn.2017.9.2.62
-
 Abstract
Abstract
 PDF
PDF Purpose
To predict the energy expenditure using the stress factor representing the ratio of the metabolic variation between pre-operation and post-operation in a pancreaticoduodenectomy (PD).
Methods
This was a prospective study conducted on 17 patients (11 males and 6 females) who underwent PD at Chonbuk National University Hospital between March 2010 and October 2011. The rest energy expenditure was measured by indirect calorimetry 1 day before and 3 days after surgery. The height, weight, and fat free mass were also measured 1 day before surgery.
Results
The mean measured rest energy expenditure 1 day before PD (mREEpre) and 3 days after PD (mREEpost) were significantly different (16.8±2.6 vs. 18.8±3.5 kcal/kg/d, P=0.0076). The stress factor, representing the ratio of the metabolic changes between pre- and post-PD, was 1.12±0.17. The recommended energy requirement for PD patients is estimated to be 23∼24 kcal/ideal body weight/d [determined from the measured preoperative rest energy expenditure (16.8±2.6 kcal/kg/d)×activity factor (1.2∼1.3)×stress factor (1.12)].
Conclusion
PD patients maintained a hypermetabolic status and the applicable stress factor was 1.12.
- 7,401 View
- 83 Download


 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN First
First Prev
Prev


