Abstract
-
Purpose
This study aimed to investigate the incidence, risk factors, and clinical course of nonalcoholic fatty liver disease (NAFLD) following pancreaticoduodenectomy, focusing on the role of adjuvant chemotherapy and other metabolic changes.
-
Methods
A retrospective analysis was conducted on 189 patients who underwent pancreaticoduodenectomy between 2013 and 2016. NAFLD was diagnosed using computed tomography (CT) imaging, defined as a liver-to-spleen attenuation ratio <0.9. Sarcopenia and sarcopenic obesity were assessed using preoperative CT scans. Logistic regression analysis was performed to identify risk factors for NAFLD development.
-
Results
The cumulative incidence of NAFLD increased over time, with rates of 15.9% at one year, 20.4% at three years, and 35.2% at five years post-pancreaticoduodenectomy. Adjuvant chemotherapy was identified as the only significant independent predictor of NAFLD development (odds ratio, 2.74; 95% confidence interval, 1.16-6.70; P=0.023). No significant associations were found between NAFLD and pancreatic enzyme replacement therapy (PERT), sarcopenia, or sarcopenic obesity. Serial analysis of NAFLD status in long-term survivors revealed dynamic changes, with some patients experiencing spontaneous remission or recurrence.
-
Conclusion
NAFLD is a common, progressive complication following pancreaticoduodenectomy, particularly in patients receiving adjuvant chemotherapy. Although no significant associations with PERT or sarcopenia were observed, these areas warrant further investigation. Long-term monitoring and targeted management strategies are recommended to address NAFLD in this population. Future prospective studies are needed to elucidate the natural history and contributing factors of NAFLD after pancreaticoduodenectomy.
-
Keywords: Exocrine pancreatic insufficiency; Non-alcoholic fatty liver disease; Pancreaticoduodenectomy; Sarcopenia
Introduction
Background/rationale
Pancreaticoduodenectomy is a standard operation for benign and malignant diseases of the pancreas head and the periampullary region. Due to the extensive and complex nature of this surgery, it has been reported with high morbidity and mortality rates [
1]. However, long-term survival after pancreaticoduodenectomy has increased not only because more patients are undergoing pancreaticoduodenectomy for benign and low-grade malignancies due to increased screening but also because of improvements in perioperative patient care and the introduction of more effective chemotherapy regimens for malignancies [
1]. This postoperative longevity has led to a growing interest in addressing long-term nutritional and metabolic issues, including nonalcoholic fatty liver disease (NAFLD) [
2-
13].
NAFLD has become one of the most widespread liver disorders globally [
14]. Its prevalence continues to grow, primarily due to rising rates of obesity, diabetes, and metabolic syndrome. Defined by the accumulation of fat within liver cells in individuals with minimal or no alcohol consumption, NAFLD has the potential to advance into nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and even hepatocellular carcinoma [
14]. Given its close association with metabolic dysfunction, NAFLD has garnered significant clinical attention.
Some previous studies have reported that pancreaticoduodenectomy is associated with the development of NAFLD, and several factors, including type of pathological disease, postoperative pancreas size, and postoperative exocrine insufficiency, have been identified as risk factors for the development of NAFLD after pancreaticoduodenectomy [
4,
5,
12,
13,
15,
16]. Alterations in bile acid metabolism, hormonal changes, and surgical disruption of the gastrointestinal and biliary systems may also contribute to changes in lipid metabolism, leading to hepatic steatosis [
17]. As most of these previous studies were conducted at a single institution with relatively small study populations and short follow-up periods, the exact pathophysiological mechanisms and contributing factors of NAFLD after pancreaticoduodenectomy have not been fully elucidated and deserve further investigation. In addition, the association of sarcopenia and sarcopenic obesity (SO) with NAFLD in the general population has been reported, but no study has addressed this issue in patients who have undergone pancreaticoduodenectomy [
18].
We conducted this study to determine the incidence and potential risk factors contributing to the development of NAFLD in patients who underwent pancreaticoduodenectomy between 2013 and 2016.
Methods
Ethics statement
This study was approved by the Institutional Review Boards of Boramae Medical Center, Seoul National University College of Medicine (IRB No. 10-2021-115). Written informed consent was waived.
Study design
Setting
This study was done at Boramae Medical Center, Samsung Medical Center, Ajou University Hospital, and Ilsan Paik Hospital between 2013 and 2016.
Participants
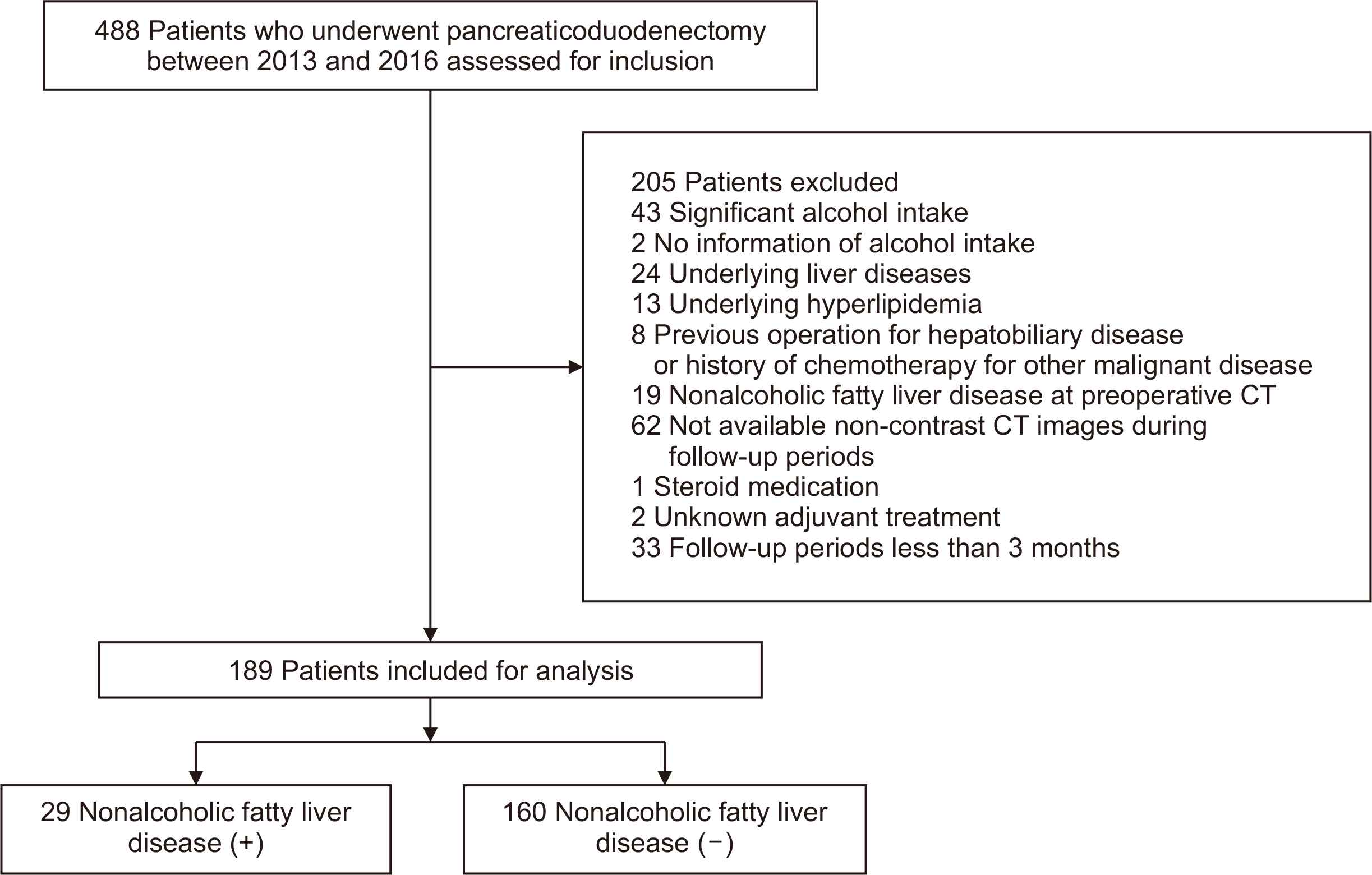
The study included 488 patients who underwent pancreaticoduodenectomy at above four hospitals between 2013 and 2016. A total of 299 patients were excluded based on the following criteria: significant alcohol intake (n=43), lack of information on alcohol intake (n=2), pre-existing liver disease (n=24), hyperlipidemia (n=13), prior hepatobiliary surgery or history of chemotherapy for other malignancies (n=8), preoperative NAFLD confirmed on computed tomography (CT) (n=19), unavailable non-contrast CT images during follow-up (n=62), steroid medication (n=1), and unknown adjuvant treatment (n=2). We also excluded the patients with insufficient follow-up duration (<12 months; n=125), because we supposed that including patients with less than 12 months of follow-up might increase the likelihood of including false-negative patients. In addition, as the study cohort included patients with poor prognosis due to malignant tumors, restricting the analysis to those with more than three or five years of follow-up would have significantly increased the number of exclusions. Therefore, to ensure a robust sample size while maintaining data accuracy, we investigated the incidence of NAFLD in patients with at least one year of follow-up and analyzed the risk factors for the occurrence of NAFLD at the one-year follow-up point after pancreaticoduodenectomy. Finally, 189 patients were included for analysis in this study (
Fig. 1).
Independent variables were age, sex, body mass index, hypertension, diabetes, alanine aminotransferase (ALT)/ aspartate aminotransferase (AST), sarcopenia, SO, presence of pancreatic head cancer, adjuvant chemotherapy, and pancreatic enzyme. The dependent variable was NAFLD.
Bias
There was no selection bias reportable.
Data sources
Data were from the patient’s medical records and CT images at four hospitals.
Measurements
To detect the development of NAFLD, liver and spleen attenuation values were measured on unenhanced CT images and presented in Housfield units. Each region of interest (ROI) was a round area with a diameter of 10 mm. For each patient, the mean attenuation value of 2 ROIs at different sectors of the liver and a single ROI of the spleen was measured. NAFLD was defined as a liver-to-spleen (L/S) attenuation ratio <0.9 [
19].
Preoperative CT images were evaluated at the portal phase of the CT scan to identify sarcopenia and SO. The diagnostic cut-off values for sarcopenia were based on the standard values which were determined in a large cohort study from a Korean national institution: skeletal muscle index (SMI=skeletal muscle area at L3/height
2)<50.18 cm
2/m
2 for males and SMI<38.63 cm
2/m
2 for females [
20]. SO was defined as visceral fat area/SMI≥2.5, derived from our previous study on the effect of SO in patients with pancreatic cancer. Detailed methods of defining sarcopenia and SO were described in our previous study [
7].
Since all target patients were recruited and included according to the selection criteria, no sample size estimation was done.
Statistical analysis
Statistical analyses were performed using R (version 4.3.3; R Foundation for Statistical Computing). Results are presented as median with interquartile range or number and percentage. Nominal variables were compared using the chi-squared test or t-test as appropriately, and continuous variables were compared using the Wilcoxon signed rank test. Logistic regression analysis was performed to determine risk factors for NAFLD, and survival analysis was performed, Kaplan-Meier analysis and log rank test. P-values of <0.05 were considered statistically significant.
Results
Clinical characteristics and risk factors of patients with and without NAFLD 1 year after pancreaticoduodenectomy
Of the 189 patients analyzed, 95 (50.3%) were male, with a median age of 64 years (57–72 years) and a median follow-up period of 35.0 months (24.0–58.0 months). All patients underwent pancreaticoduodenectomy for pancreatic head cancer (70, 37.0%), distal bile common duct cancer (55, 29.1%), ampulla of Vater cancer (40, 21.2%), intraductal papillary mucinous neoplasm (11, 5.8%), duodenal cancer (6, 3.2%), SPN (2, 1.1%), and other diagnoses (5, 2.6%).
At one year after pancreaticoduodenectomy, 29 patients (15.3%) were classified as NAFLD-positive (NAFLD+), while 160 (84.7%) remained NAFLD-negative (NAFLD–) (
Table 1). No statistically significant differences were observed between the NAFLD+ and NAFLD– groups in terms of median age (64 years vs. 65 years, P=0.096) or body mass index (BMI) (23.5 kg/m
2 vs. 23.6 kg/m
2, P=0.969), AST levels (30.0 U/L vs. 32.5 U/L, P=0.976), or ALT levels (48.0 U/L vs. 43.0 U/L, P=0.589). Although a higher percentage of females were in the NAFLD+ group (58.6%) compared to males (48.1%), this difference was not statistically significant (P=0.402). Sarcopenia (27.6% vs. 18.8%, P=0.401) and SO (51.7% vs. 43.8%, P=0.554) were slightly more prevalent in the NAFLD+ group compared to the NAFLD– group; however, these differences did not reach statistical significance.
The proportion of patients undergoing pancreaticoduodenectomy for pancreatic head cancer was comparable between the groups, as were the operative factors. The clinically relevant postoperative pancreatic fistula occurred similarly in both groups, but higher-grade postoperative complications (Clavien-Dindo grade IIIA or higher) occurred more frequently in the NAFLD+ group (34.5% vs. 21.9%, P=0.219), although this difference was not statistically significant. The administration of adjuvant chemotherapy was more prevalent in the NAFLD+ group (58.6%) than in the NAFLD– group (40.0%), with a trend towards significance (P=0.097). The median duration of pancreatic enzyme supplementation was 2.8 months (0.7–10.5 months), with a median of 3.7 months (0.7–11.2 months) in the NAFLD+ group and 2.5 months (0.7–10.2 months) in the NAFLD– group. This difference was also not statistically significant (P=0.456).
Univariate and multivariate logistic regression analyses were performed to assess predictors of NAFLD one year after pancreaticoduodenectomy (
Table 2). Univariate analysis showed a trend towards a higher risk of NAFLD in patients receiving adjuvant chemotherapy (odds ratio [OR], 2.13; 95% confidence interval [CI], 0.96–4.85; P=0.066). On multivariate analysis, adjuvant chemotherapy was identified as a significant independent predictor of NAFLD (OR, 2.74; 95% CI, 1.16–6.70; P=0.023). Other factors such as age, sex, BMI, hypertension, diabetes, ALT/AST ratio, sarcopenia, and SO did not reach statistical significance in the multivariate model.
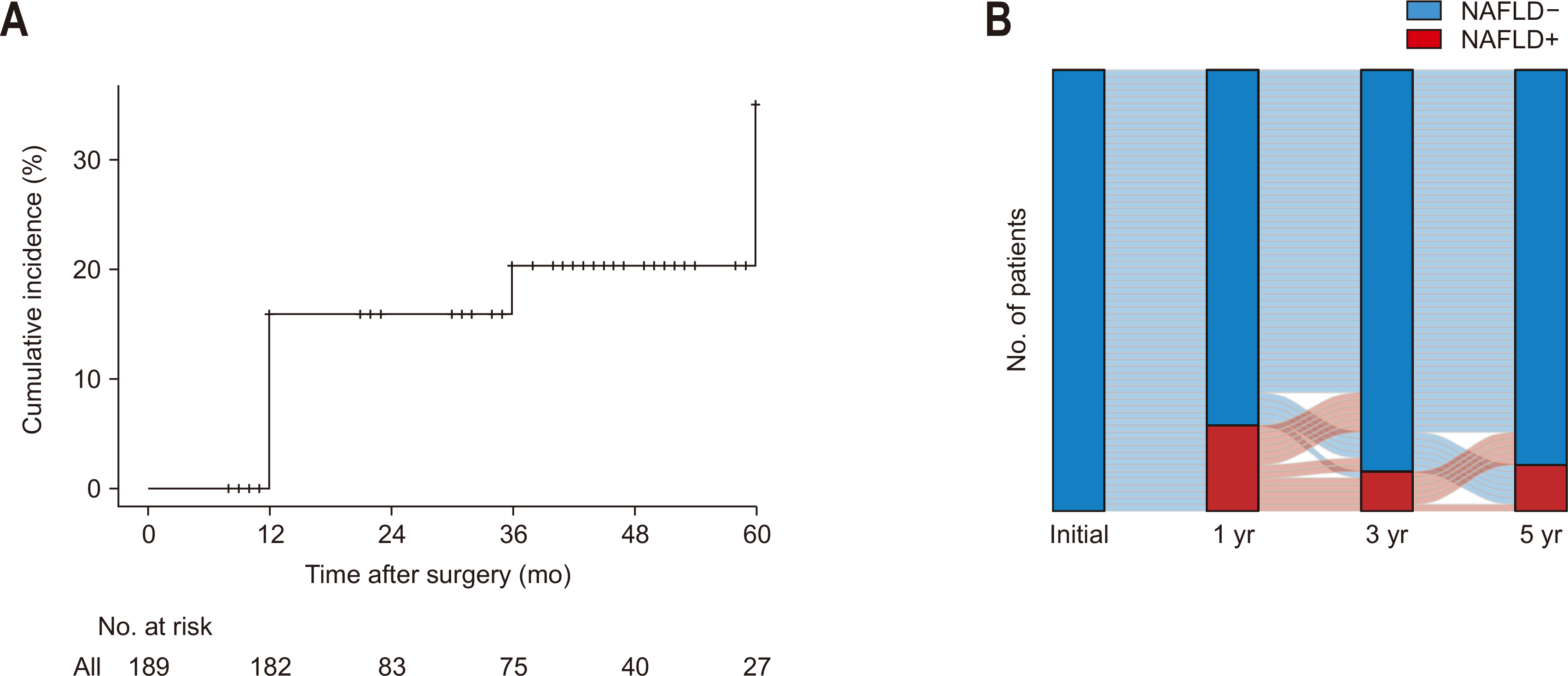
The cumulative incidence of NAFLD following pancreaticoduodenectomy increased over time with rates of 15.9% at one year, 20.4% at three years, and 35.2% at five years (
Fig. 2A). During the follow-up period, no patients diagnosed with NAFLD progressed to NASH or cirrhosis. We analyzed the serial change in NAFLD status among 67 patients who survived for a minimum of five years to investigate the dynamic nature of NAFLD (
Fig. 2B). We analyzed the NAFLD status of 67 patients who survived more than 5 years to explore the dynamic nature of NAFLD (
Fig. 2B). At the one-year follow-up, 13 patients (19.4%) had developed NAFLD. Among these patients, 6 experienced spontaneous NAFLD remission at three year after pancreaticoduodenectomy, which was maintained through the 5-year follow-up. Another 2 patients improved at 3 years but experienced a recurrence by the five-year follow-up, and 4 patients demonstrated improvement of NAFLD only at five years after pancreaticoduodenectomy. One patient had persistent NAFLD throughout the follow-up period.
The entire cohort of 281 patients, including those initially excluded based on follow-up duration, comprised 141 males with a median age of 63 years (57–73 years) and a median follow-up period of 35 months (17–51 months). The prevalence of NAFLD was observed at 11.7% (26/222) at three months, 15.3% (29/189) at 12 months, 9.5% (12/126) at 36 months, and 12.5% (12/96) at 60 months postoperatively.
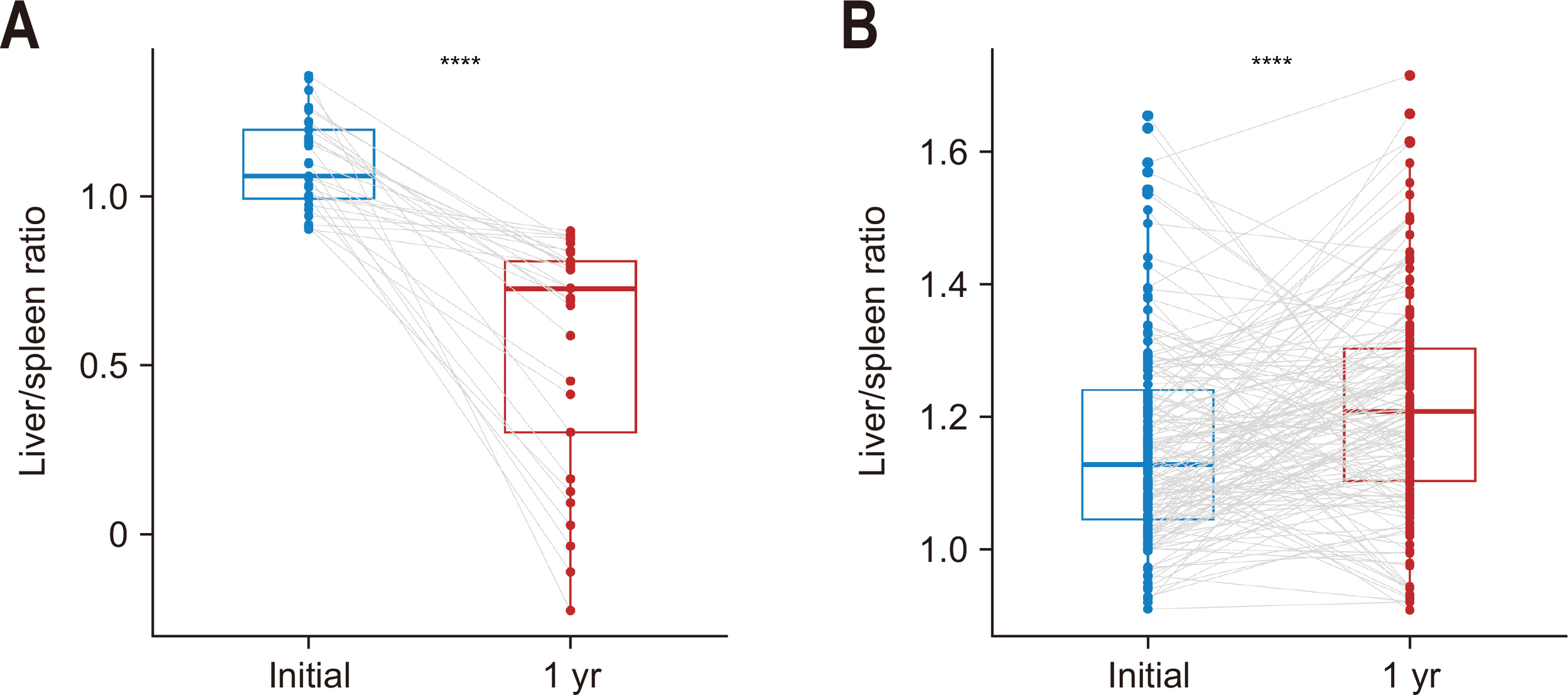
Changes in the L/S attenuation ratio from preoperative to 1 year postoperative were examined in patients with and without NAFLD.
Fig. 3A shows the alteration in L/S ratio among NAFLD+ patients, exhibiting a significant decrease in median L/S ratio from 1.06 (0.99–1.20) preoperatively to 0.73 (0.30–0.81) at one year (P<0.001). In contrast, patients without NAFLD showed a modest increase in the L/S ratio from 1.13 (1.05–1.24) preoperatively to 1.21 (1.10–1.30) at the one-year mark (P<0.001;
Fig. 3B).
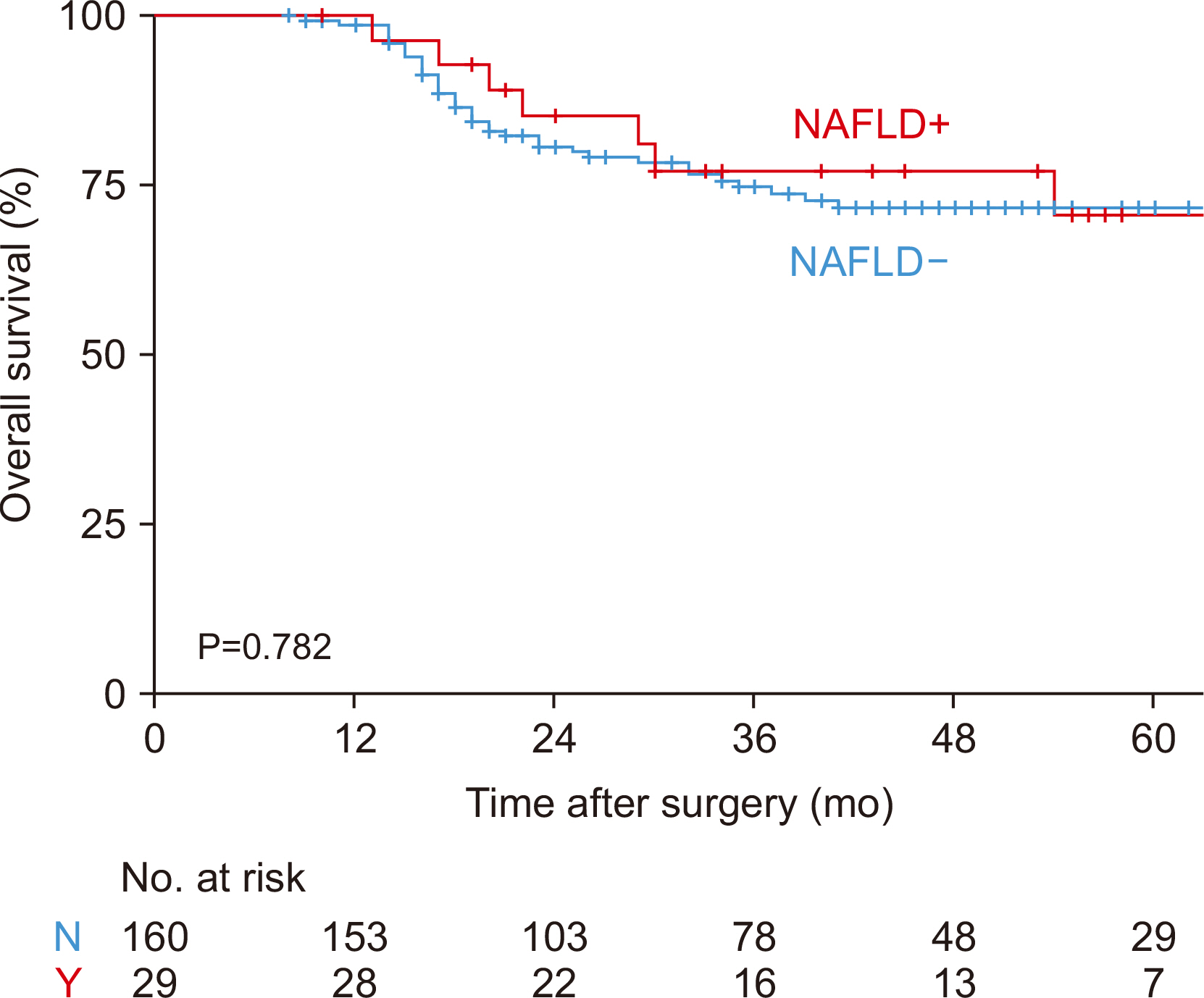
The one-, three-, and five-year overall survival (OS) rates for patients with NAFLD were 100.0%, 85.3%, and 70.7% respectively (
Fig. 4). The corresponding figures for the patients without NAFLD were 98.7%, 74.9%, and 71.8%, respectively. No significant statistical difference was observed in OS between patients with and without NAFLD (P=0.782).
Discussion
Key results
The present study identified the cumulative incidence of NAFLD following pancreaticoduodenectomy increased over time, with rates of 15.9% at one year to 35.2% at five years, highlighting the need for long-term surveillance. Also, adjuvant chemotherapy was identified as a key factor in NAFLD development after pancreaticoduodenectomy.
Interpretation/comparison with previous studies
These results align with previous studies that have reported NAFLD incidence rates ranging from 7.8% to 37.5% following pancreaticoduodenectomy [
11,
16]. We noticed changes in NAFLD prevalence over time, consistent with previous studies reporting improvements in NAFLD with pancreatic enzyme supplementation therapy or cessation of chemotherapy [
4,
6,
11,
15,
16]. This dynamic pattern suggests the presence of unique contributing factors for post-pancreaticoduodenectomy NAFLD, distinct from those associated with NAFLD in the general population, such as obesity and metabolic syndrome. It implies that targeted management may potentially reduce NAFLD occurrences after pancreaticoduodenectomy.
Our study, identifing adjuvant chemotherapy as a key factor in NAFLD development after pancreaticoduodenectomy, is consistent with a previous study by Nishikawa et al. [
6], which reported a higher incidence of NAFLD in patients receiving adjuvant chemotherapy (38% vs. 19%, P=0.016). Chemotherapy-induced oxidative stress is thought to play a role in this mechanism, potentially leading to mitochondrial dysfunction in hepatocytes and subsequent fat accumulation in the liver. Factors such as the duration of chemotherapy, the timing between chemotherapy and surgery, the pharmacogenetics of chemotherapeutic agents, as well as patient-specific characteristics such as age, sex, nutritional status, underlying comorbidities, and genetic susceptibility may all influence the development of NAFLD after chemotherapy [
21]. However, the relationship between adjuvant chemotherapy and the development of NAFLD after pancreaticoduodenectomy remains controversial. Several studies have reported that chemotherapy was not significantly associated with the occurrence of NAFLD after pancreaticoduodenectomy [
4,
5,
8,
9]. This discrepancy in findings suggests that the development of NAFLD following pancreaticoduodenectomy is likely a multifactorial process involving complex interactions between chemotherapy and other risk factors. Jung et al. [
3] reported that remnant pancreas atrophy was more severe in patients with malignant disease who received adjuvant chemoradiotherapy and was associated with pancreatic exocrine and endocrine insufficiency.
Similarly, Shin et al. [
10] found that nutritional status indicators such as relative body weight, BMI, and triceps skinfold thickness only begin to recover 6 months post-surgery and typically require 12 months for full recovery. They also noted that adjuvant therapy was related to exocrine function in univariate analysis and had a significantly impacted endocrine insufficiency. Therefore, further research is needed to elucidate the intricate relationships between these factors and their collective impact on NAFLD development.
The present study did not show the association of pancreatic enzyme replacement therapy (PERT) with NAFLD. Since Tanaka et al. [
12] first reported a significant improvement in hepatic steatosis with higher doses of pancreatic enzyme supplementation, and Nakagawa et al. [
5] initially reported a close association between postoperative pancreatic exocrine insufficiency (PEI) and NAFLD development after pancreaticoduodenectomy, several subsequent studies have also demonstrated benefits of high-dose PERT in reducing NAFLD incidence and improving existing NAFLD [
4,
13,
15]. The possible mechanism by which PEI leads to NAFLD after pancreaticoduodenectomy involves decreased digestive enzyme secretion, resulting in malabsorption of essential nutrients and fatty acids, which disrupts hepatic lipid metabolism and triggers compensatory hepatic lipogenesis, ultimately causing fat accumulation in the liver [
17,
22]. However, conflicting results have been reported by D’Cruz et al. [
23], who found that 31% of patients developed NAFLD despite 95% receiving enzyme supplementation, and another study by Sato et al. [
8] showing no significant difference in NAFLD incidence between patients who did or did not receive prophylactic PERT supplementation. A randomized controlled trial by Satoi et al. [
9] also failed to identify a protective effect of pancrealipase replacement therapy over conventional pancreatic enzyme supplementation on NAFLD development after pancreaticoduodenectomy. These discrepancies may be attributed to differences in dosing regimens, timing of PERT initiation, patient compliance, or the severity of PEI across cohorts. The conflicting findings underscore the multifactorial nature of post-pancreatectomy NAFLD and suggest that PERT alone may not be sufficient for prevention or treatment in all patients.
Our research didn’t show a clear statistical link between sarcopenia, SO, and NAFLD, but we noticed these conditions were more common in patients with NAFLD. The incidence of preoperative sarcopenia and SO in patients undergoing pancreaticoduodenectomy has been reported to range from 25.1% to 67.3% and 24.2% to 36.9%, respectively, and both conditions are linked to increased postoperative complications and poorer long-term survival [
7,
24-
26]. Studies on the general population have shown that sarcopenia and SO are closely associated with the development of NAFLD as well as related morbidity and mortality [
18,
26-
29]. The mechanisms underlying the development of NAFLD in patients with sarcopenia and SO are multifactorial and are believed to involve pathophysiologic processes such as insulin resistance, inflammatory cytokine release, and oxidative stress [
18,
27]. Our study could not find such associations, because the complex metabolic changes following pancreaticoduodenectomy might influence the relationship between sarcopenia and NAFLD in a different way than in the general population. It’s also possible our sample size was too small to detect these relationships. To the best of our knowledge, no previous studies have specifically investigated the relationship between sarcopenia, SO, and NAFLD after pancreaticoduodenectomy. The complex interplay between muscle, adipose tissue, and the liver in the post-pancreaticoduodenectomy patients is still poorly understood and further research is warranted.
First, although this study analyzed a relatively large multicenter cohort, the excluding patients with shorter follow-up durations and those with poor prognosis may have introduced selection bias, potentially underestimating the incidence of NAFLD during the early postoperative period. Second, the lack of standardized protocols for PERT and adjuvant chemotherapy across institutions contributed to heterogeneity in the data. Third, the diagnosis of NAFLD was based on CT imaging rather than liver biopsy, and it may not capture all cases of NAFLD, particularly in its early stages. However, we used CT scan for diagnosis of NAFLD, as in most other previous studies, because CT-based diagnosis is widely used in clinical practice and research and has been reported to have a sensitivity of 82%–93% and a specificity of 100% in the cases with more than 30% hepatic steatosis [
30,
31]. Finally, we could not account for all potential confounding factors that could influence NAFLD development, such as detailed dietary habits and physical activity levels. Future studies with prospective designs and comprehensive data on these lifestyle factors are needed to address these limitations and validate our findings.
Our study demonstrates that NAFLD is a common and progressive complication following pancreaticoduodenectomy, with adjuvant chemotherapy emerging as a significant risk factor. We did not find strong associations between pancreatic enzyme supplementation or sarcopenia and NAFLD; however, these areas deserve further investigation. Careful monitoring of liver status in patients who have undergone pancreaticoduodenectomy is necessary, especially in patients underwent adjuvant chemotherapy. Further prospective research with extended follow-up durations is essential to understand better the progression and contributing factors of NAFLD in this population.
Acknowledgments
The authors thank Hyemin Kim (Data Manager, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine) for help with data collection.
Authors’ contribution
Conceptualization: CSL, HK, IWH, YCS, WJ. Data curation: IWH, CSL, HK, YCS, EG, JL, KCY, SJY, SHS, JSH, WJ. Formal analysis: CSL, HK, IWH, WGY, EG, JL, KCY, SJY, SHS, JSH, WJ. Funding acquisition: CSL, HK, IWH, YCS, WJ. Investigation: EG, JL, KCY, SJY, SHS, JSH. Methodology: CSL, HK, IWH, YCS, WJ. Project administration: EG, JL, KCY, SJY, SHS, JSH. Resources: CSL, HK, IWH, YCS, WJ. Supervision: WGY, EG, JL, KCY, SJY, SHS, JSH. Visualization: CSL, WGY, EG, JL, SJY. Writing – original draft: CSL, HK, WGY. Writing – review and editing: all authors.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
This study was supported by the 2021 Korean Society of Surgical Metabolism and Nutrition Research Grant. (No. 2021-003)
Data availability
Contact the corresponding author for data availability.
Supplementary materials
None.
Fig. 1Flow chart of patient selection for the present study. CT = computed tomography.
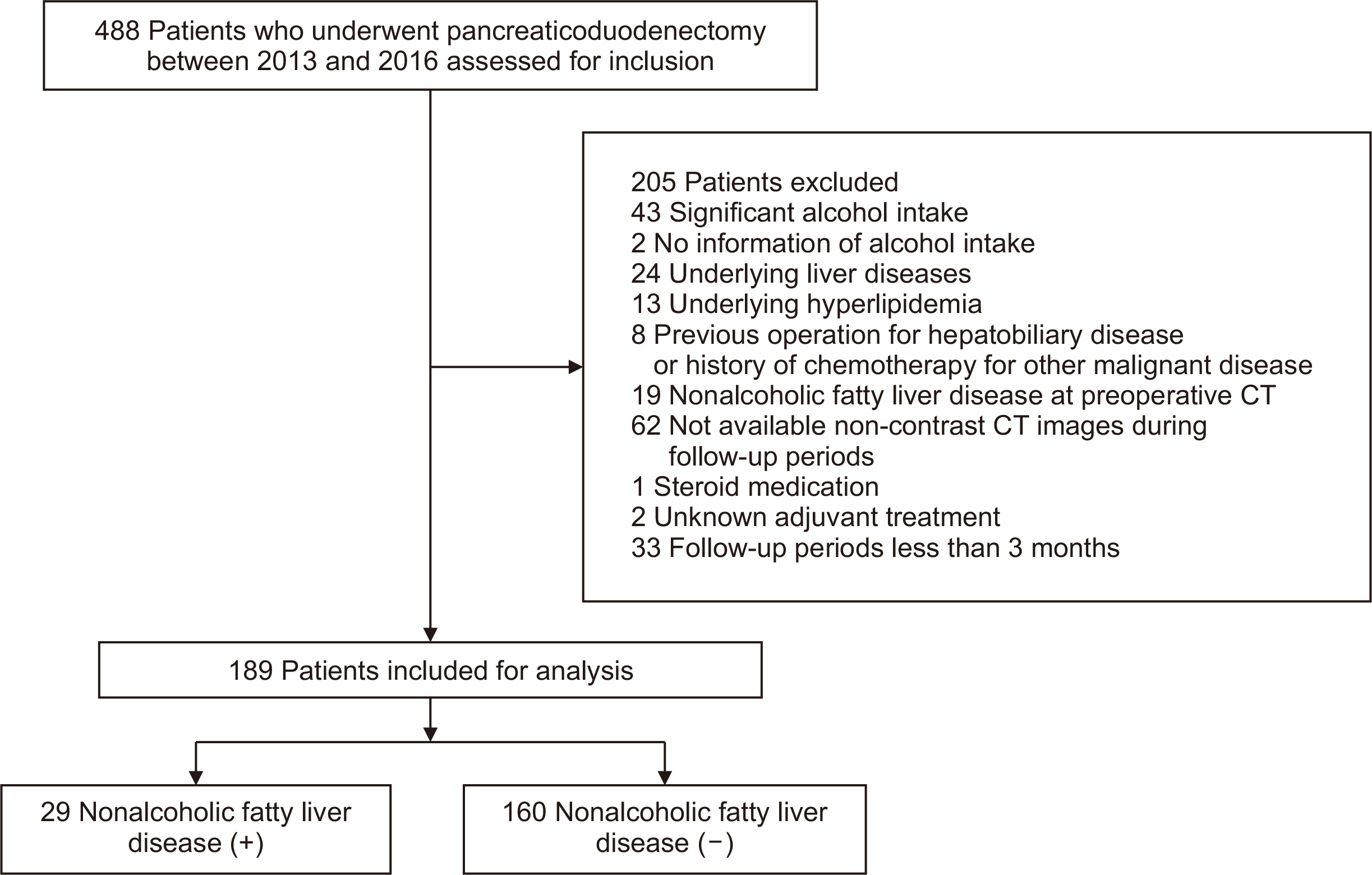
Fig. 2Incidence of nonalcoholic fatty liver disease (NAFLD) after pancreaticoduodenectomy. (A) The cumulative incidence rates for the entire study population (n=189) were 15.9%, 20.4%, and 35.2%, at 1, 3, and 5 years postoperatively, respectively. (B) Serial changes of NAFLD status in patients who survived more than 5 years.
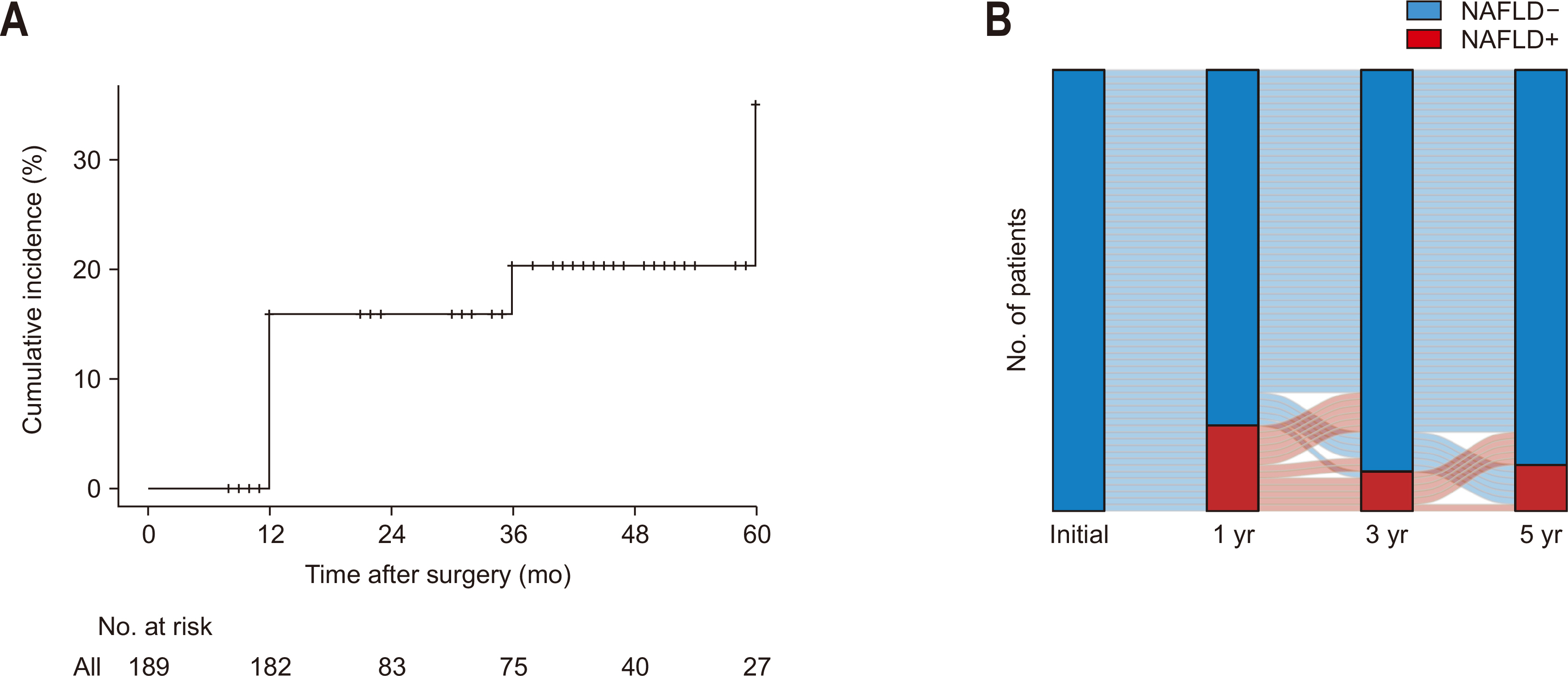
Fig. 3Changes of liver-to-spleen ratio in patients with nonalcoholic fatty liver disease (NAFLD) (A) and those without NAFLD (B) at the timing of 1 year after pancreaticoduodenectomy. ****P<0.001.
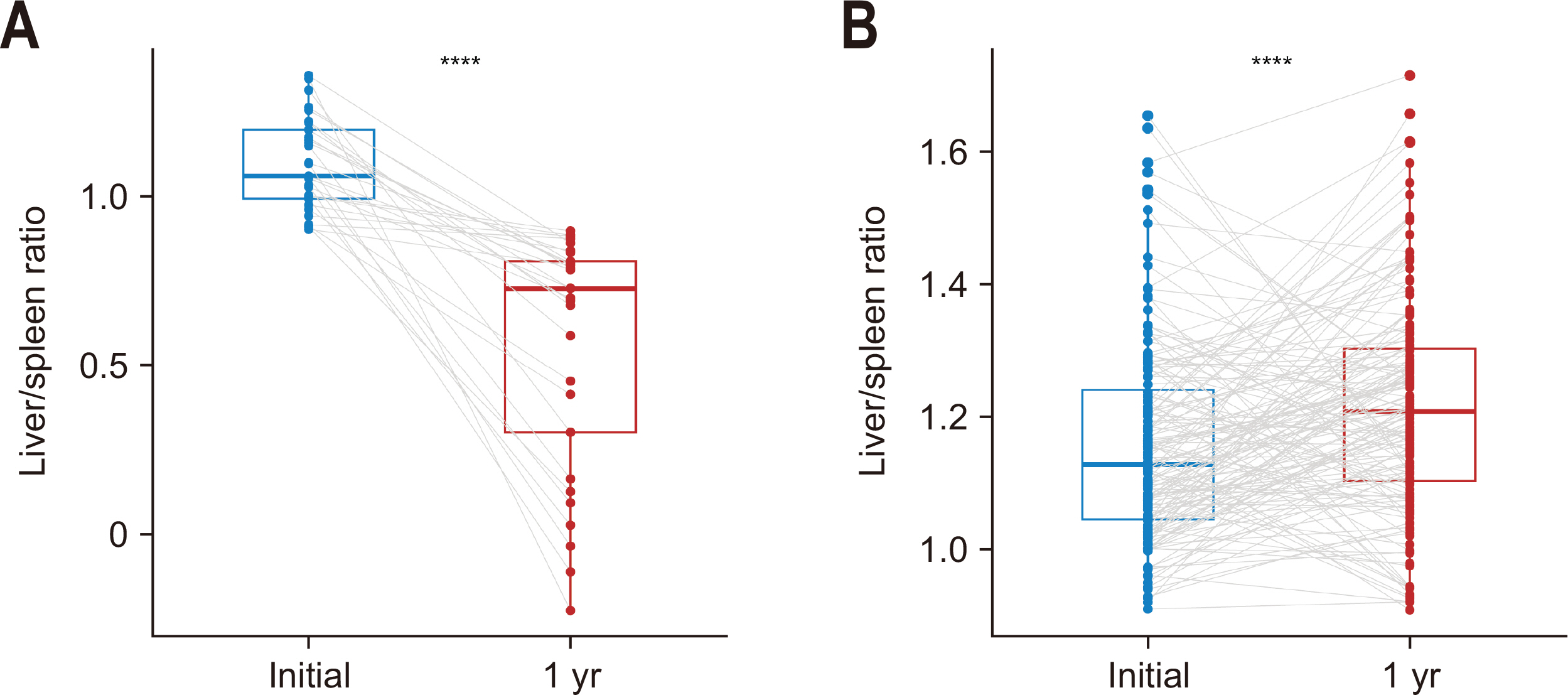
Fig. 4Overall survival according to the occurrence of nonalcoholic fatty liver disease (NAFLD). N = no; Y = yes.
Table 1Clinical characteristics between patients with NAFLD and those without NAFLD at the timing of 1 year after PD
|
Total |
NAFLD+ |
NAFLD– |
P-value |
|
Number |
189 (100.0) |
29 (15.3) |
160 (84.7) |
|
|
Age (yr)>60 |
119 (63.0) |
14 (48.3) |
105 (65.6) |
0.335 |
|
Sex |
|
|
|
0.402 |
|
Female |
94 (49.7) |
17 (58.6) |
77 (48.1) |
|
|
Male |
95 (50.3) |
12 (41.4) |
83 (51.9) |
|
|
Body mass index (kg/m2)≥23 |
109 (57.7) |
16 (55.2) |
93 (58.1) |
0.927 |
|
Hypertension (Y) |
76 (40.2) |
10 (34.5) |
66 (41.3) |
0.633 |
|
Diabetes (Y) |
57 (30.2) |
6 (20.7) |
51 (31.9) |
0.323 |
|
ALT/AST≥1.33 |
73 (38.6) |
14 (48.3) |
59 (36.9) |
0.341 |
|
Sarcopenia (Y) |
38 (20.1) |
8 (27.6) |
30 (18.8) |
0.401 |
|
Sarcopenic obesity (Y) |
85 (45.0) |
15 (51.7) |
70 (43.8) |
0.554 |
|
Diagnosis |
|
|
|
0.604 |
|
PHC |
70 (37.0) |
9 (31.0) |
61 (38.1) |
|
|
Others |
119 (63.0) |
20 (69.0) |
99 (61.9) |
|
|
Operation time (min)a
|
313 (278–360) |
313 (265–360) |
314 (279–358) |
0.981 |
|
Blood loss (mL)a
|
350 (250–500) |
300 (250–600) |
350 (250–500) |
0.747 |
|
Complication (≥IIIa) |
45 (23.8) |
10 (34.5) |
35 (21.9) |
0.219 |
|
CR-POPF |
24 (12.7) |
4 (13.8) |
20 (12.5) |
0.768 |
|
Adjuvant chemotherapy (Y) |
81 (42.9) |
17 (58.6) |
64 (40.0) |
0.097 |
|
Pancreatic enzyme≥1Y |
43 (22.8) |
6 (20.7) |
37 (23.1) |
0.962 |
Table 2Predictive factors for NALFD at the timing of 1 year after PD
|
Variable |
Univariate analysis |
|
Multivariate analysis |
|
|
|
OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
|
Age (yr) |
|
|
|
|
|
|
≤60 vs. >60 |
0.49 (0.22–1.09) |
0.079 |
|
NA |
NA |
|
Sex |
|
|
|
|
|
|
Female vs. Male |
0.65 (0.29–1.45) |
0.301 |
|
NA |
NA |
|
Body mass index (kg/m2) |
|
|
|
|
|
|
<23 vs. ≥23 |
0.89 (0.40–1.99) |
0.767 |
|
NA |
NA |
|
Hypertension |
|
|
|
|
|
|
N vs. Y |
0.75 (0.32–1.68) |
0.495 |
|
NA |
NA |
|
Diabetes |
|
|
|
|
|
|
N vs. Y |
0.56 (0.20–1.38) |
0.232 |
|
NA |
NA |
|
ALT/AST |
|
|
|
|
|
|
<1.33 vs. ≥1.33 |
1.60 (0.71–3.56) |
0.249 |
|
NA |
NA |
|
Sarcopenia |
|
|
|
|
|
|
N vs. Y |
1.65 (0.64–3.97) |
0.278 |
|
NA |
NA |
|
Sarcopenic obesity |
|
|
|
|
|
|
N vs. Y |
1.38 (0.62–3.07) |
0.428 |
|
NA |
NA |
|
Diagnosis |
|
|
|
|
|
|
Others vs. PHC |
0.73 (0.30–1.67) |
0.468 |
|
0.49 (0.19–1.21) |
0.132 |
|
Adjuvant chemotherapy |
|
|
|
|
|
|
N vs. Y |
2.13 (0.96–4.85) |
0.066 |
|
2.74 (1.16–6.70) |
0.023 |
|
Pancreatic enzyme |
|
|
|
|
|
|
<1Y vs. ≥1Y |
0.87 (0.30–2.17) |
0.774 |
|
NA |
NA |
References
- 1. Byun Y, Choi YJ, Han Y, Kang JS, Kim H, Kwon W, et al. Outcomes of 5000 pancreatectomies in Korean single referral center and literature reviews. J Hepatobiliary Pancreat Sci 2022;29:1327-35. ArticlePubMedPDF
- 2. Huang K, Qian T, Chen W, Bai X, Gao S, Shen Y, et al. Clinical significance and risk factors of nonalcoholic fatty liver diseases after whipple procedure. J Surg Res 2024;302:706-14. ArticlePubMed
- 3. Jung W, Kim H, Kwon W, Jang JY. Atrophy of remnant pancreas after pancreatoduodenectomy: risk factors and effects on quality of life, nutritional status, and pancreatic function. J Hepatobiliary Pancreat Sci 2022;29:239-49. ArticlePubMedPDF
- 4. Nagai M, Sho M, Satoi S, Toyokawa H, Akahori T, Yanagimoto H, et al. Effects of pancrelipase on nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2014;21:186-92. ArticlePubMed
- 5. Nakagawa N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, et al. Nonalcoholic fatty liver disease after pancreatoduodenectomy is closely associated with postoperative pancreatic exocrine insufficiency. J Surg Oncol 2014;110:720-6. ArticlePubMedPDF
- 6. Nishikawa M, Aosasa S, Moriya T, Noro T, Hase K, Yamamoto J. The impact of postoperative adjuvant chemotherapy on the development of nonalcoholic fatty liver disease after pancreatoduodenectomy. J Surg Res 2016;205:127-35. ArticlePubMed
- 7. Ryu Y, Shin SH, Kim JH, Jeong WK, Park DJ, Kim N, et al. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB (Oxford) 2020;22:1782-92. ArticlePubMed
- 8. Sato T, Matsuo Y, Shiga K, Morimoto M, Miyai H, Takeyama H. Factors that predict the occurrence of and recovery from non-alcoholic fatty liver disease after pancreatoduodenectomy. Surgery 2016;160:318-30. ArticlePubMed
- 9. Satoi S, Sho M, Yanagimoto H, Yamamoto T, Akahori T, Kinoshita S, et al. Do pancrelipase delayed-release capsules have a protective role against nonalcoholic fatty liver disease after pancreatoduodenectomy in patients with pancreatic cancer? A randomized controlled trial. J Hepatobiliary Pancreat Sci 2016;23:167-73. ArticlePubMed
- 10. Shin YC, Han Y, Kim E, Kwon W, Kim H, Jang JY. Effects of pancreatectomy on nutritional state, pancreatic function, and quality of life over 5 years of follow up. J Hepatobiliary Pancreat Sci 2022;29:1175-84. ArticlePubMedPDF
- 11. Song SC, Choi SH, Choi DW, Heo JS, Kim WS, Kim MJ. Potential risk factors for nonalcoholic steatohepatitis related to pancreatic secretions following pancreaticoduodenectomy. World J Gastroenterol 2011;17:3716-23. ArticlePubMedPMC
- 12. Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, et al. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol 2011;46:758-68. ArticlePubMedPDF
- 13. Yasukawa K, Shimizu A, Yokoyama T, Kubota K, Notake T, Seki H, et al. Preventive effect of high-dose digestive enzyme management on development of nonalcoholic fatty liver disease after pancreaticoduodenectomy: a randomized controlled clinical trial. J Am Coll Surg 2020;231:658-69. ArticlePubMed
- 14. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020;158:1851-64. ArticlePubMed
- 15. Yamazaki S, Takayama T, Higaki T, Moriguchi M, Yoshida N, Miyazaki T, et al. Pancrelipase with branched-chain amino acids for preventing nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Gastroenterol 2016;51:55-62. ArticlePubMedPDF
- 16. Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, et al. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci 2010;17:296-304. ArticlePubMedPDF
- 17. Kang CM, Lee JH. Pathophysiology after pancreaticoduodenectomy. World J Gastroenterol 2015;21:5794-804. ArticlePubMedPMC
- 18. Polyzos SA, Vachliotis ID, Mantzoros CS. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 2023;147:155676. ArticlePubMed
- 19. Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, et al. 1997;Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol 27:108-13. ArticlePubMed
- 20. Moon JH, Kim KM, Kim JH, Moon JH, Choi SH, Lim S, et al. predictive values of the new sarcopenia index by the foundation for the national institutes of health sarcopenia project for mortality among older Korean adults. PLoS One 2016;11:e0166344. ArticlePubMedPMC
- 21. Meunier L, Larrey D. 2020;Chemotherapy-associated steatohepatitis. Ann Hepatol 19:597-601. ArticlePubMed
- 22. Shah P, Patel V, Ashkar M. De novo non-alcoholic fatty liver disease after pancreatectomy: a systematic review. World J Clin Cases 2022;10:12946-58. ArticlePubMedPMC
- 23. D'Cruz V, De Zutter A, Van den Broecke M, Ribeiro S, Abreu de Carvalho L, Smeets P, et al. Prevalence of metabolic dysfunction-associated fatty liver disease after pancreatic surgery in a historical Belgian cohort and review of the literature. Acta Gastroenterol Belg 2024;87:373-80. ArticlePubMed
- 24. Ciesielka J, Jakimów K, Majewska K, Mrowiec S, Jabłońska B. The association between preoperative sarcopenia and sarcopenic obesity and the occurrence of postoperative complications in patients undergoing pancreaticoduodenectomy for periampullary malignancies-a literature review. Nutrients 2024;16:3569.ArticlePubMedPMC
- 25. Zhang QH, Ma JD, Lu YM, Zhang RN, Zhao ZH, Li YT, et al. Sarcopenia adversely impacts clinical outcomes in patients undergoing pancreaticoduodenectomy: a systematic review and meta-analysis. World J Gastrointest Surg 2024;16:1857-70. ArticlePubMedPMC
- 26. Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr 2019;19:183.ArticlePubMedPMCPDF
- 27. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772-8. ArticlePubMed
- 28. Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol 2015;63:486-93. ArticlePubMed
- 29. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123-31. ArticlePubMed
- 30. Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol 2008;7:212-20. ArticlePubMed
- 31. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745-50. ArticlePubMed

 , Hongbeom Kim2
, Hongbeom Kim2 , In Woong Han2
, In Woong Han2 , Won-Gun Yun1
, Won-Gun Yun1 , Eunchae Go1
, Eunchae Go1 , Jaewon Lee1
, Jaewon Lee1 , Kyung Chul Yoon1
, Kyung Chul Yoon1 , So Jeong Yoon2
, So Jeong Yoon2 , Sang Hyun Shin2
, Sang Hyun Shin2 , Jin Seok Heo2
, Jin Seok Heo2 , Yong Chan Shin3
, Yong Chan Shin3 , Woohyun Jung4
, Woohyun Jung4





 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 Cite
Cite

