Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > J Clin Nutr > Volume 11(1); 2019 > Article
- Original Article Evaluation of the Clinical Effect of Intravenous Glutamine on Intensive Care Unit Patients
-
Kwang Joon Kim
 , Hey Young Jang, Min Ku Kang
, Hey Young Jang, Min Ku Kang - 중환자실 환자의 정맥주사용 글루타민 투여에 따른 임상효과 평가
-
김광준
 , 장혜영, 강민구
, 장혜영, 강민구 -
Journal of the Korean Society for Parenteral and Enteral Nutrition 2019;11(1):23-28.
DOI: https://doi.org/10.15747/jcn.2019.11.1.23
Published online: June 30, 2019
Department of Pharmacy, Chosun University Hospital, Gwangju, Korea
- Correspondence to Kwang Joon Kim Department of Pharmacy, Chosun University Hospital, 365 Pilmun-daero, Dong-gu, Gwangju 61453, KoreaTel: +82-62-220-3296, Fax: +82-62-227-3745, E-mail: nocky@hanmail.net
Copyright: © Korean Society for Parenteral and Enteral Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 585 Views
- 3 Download
Abstract
-
Purpose: To evaluate the clinical effect of intravenous glutamine administration on patients admitted to the intensive care unit in general hospitals.
-
Methods: Patients with more than 7 days in an intensive care unit were evaluated. The experimental group was the patients who received intravenous glutamine administration for more than 3 days. The laboratory results, intensive care unit length of stay, hospital length of stay, 30 days mortality, and hospital mortality were evaluated with a comparative group.
-
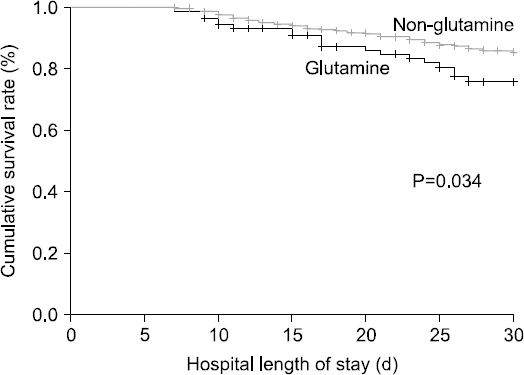
Results: The mean number of administration days of intravenous glutamine was 10.12±8.93 days, and the average daily dose was 0.33±0.10 g/kg/day. No adequate improvement in the laboratory results of glutamine-treated group was observed. The intensive care unit length of stay (21.16±15.83 vs. 16.48±11.06, P=0.007), hospital length of stay (35.94±30.75 vs. 27.34±19.09, P=0.010), 30 days mortality (20.0% vs. 10.0%, P=0.034), and hospital mortality (26.3% vs. 13.0%, P=0.001) were higher in the glutamine-treated group.
-
Conclusion: The use of intravenous glutamine on intensive care unit patients did not improve the clinical effect. Further large-scale multi-center studies will be needed to assess the proper administration of intravenous glutamine on intensive care unit patients.
서 론
대상 및 방법
결 과
| Characteristic | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| Gender | 0.296 | ||
| Male | 63 (66.3) | 341 (60.7) | |
| Female | 32 (33.7) | 221 (39.3) | |
| Age (y) | 66.11±15.78 | 67.75±15.47 | 0.341 |
| Height (cm) | 166.57±8.62 | 163.61±10.08 | 0.008** |
| Weight (kg) | 61.35±12.15 | 61.38±12.77 | 0.984 |
| Admission type | 0.000*** | ||
| NS, NR | 20 (21.1) | 256 (45.6) | |
| GS, GE | 18 (18.9) | 44 (7.8) | |
| HE | 1 (1.1) | 3 (0.5) | |
| RP | 42 (44.2) | 113 (20.1) | |
| NH, UR | 1 (1.1) | 43 (7.7) | |
| Etc. | 13 (13.7) | 103 (18.3) | |
| APACHE II score at ICU entry | 6.00±7.36 | 6.26±6.58 | 0.720 |
Values are presented as number (%) or mean±standard deviation.
NS = neurosurgery; NR = neurology; GS = general surgery; GE = gastroenterology; HE = hematology; RP = respiratory medicine; NH = nephrology; UR = urology; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
**P<0.01,
***P<0.001.
| Glutamine administration (L-alanyl-L-glutamine) | Glutamine for 3 days or more (n=95) |
|---|---|
| Day | 10.12±8.93 |
| Dose (g/kg/d)a | 0.33±0.10 |
Values are presented as mean±standard deviation.
aGuideline statement of intravenous glutamine supplementation dose: 0.3∼0.6 g/kg/day L-alanyl-L-glutamine.9
| Laboratory result | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| Serum creatinine (mg/dL) | 2.48±11.25 | 2.08±7.48 | 0.660 |
| Blood urea nitrogen (mg/dL) | 29.06±33.07 | 26.26±25.48 | 0.346 |
| Aspartate aminotransferase (U/L) | 90.15±295.13 | 123.44±574.60 | 0.581 |
| Alanine transaminase (U/L) | 63.50±190.39 | 67.29±287.76 | 0.902 |
| Total bilirubin (mg/dL) | 1.46±4.15 | 0.89±1.78 | 0.186 |
| Albumin (g/dL) | 3.12±0.84 | 3.41±0.78 | 0.001** |
| Total protein (g/dL) | 5.77±1.00 | 6.04±1.00 | 0.020* |
| C-reactive protein (mg/dL) | 11.48±10.77 | 9.56±9.76 | 0.085 |
| Glucose (mg/dL) | 151.43±62.86 | 158.83±95.53 | 0.334 |
| Triglyceride (mg/dL) | 116.4±76.95 | 118.83±99.33 | 0.825 |
| Laboratory result | Glutamine for 3 days or more (n=95) | P-value | Non-glutamine (n=562) | P-value | P-value | |
|---|---|---|---|---|---|---|
| Serum creatinine (mg/dL) | Pre | 2.47±11.25 | 0.329 | 2.09±7.52 | 0.012* | 0.062 |
| Post | 1.33±1.44 | 0.329 | 1.26±1.70 | |||
| Blood urea nitrogen (mg/dL) | Pre | 29.06±33.07 | 0.128 | 26.24±25.64 | 0.048* | 0.022* |
| Post | 35.27±28.21 | 0.128 | 29.24±28.29 | |||
| Aspartate aminotransferase (U/L) | Pre | 90.15±295.13 | 0.972 | 124.64±578.72 | 0.065 | 0.283 |
| Post | 88.92±188.73 | 0.972 | 73.42±294.86 | |||
| Alanine transaminase (U/L) | Pre | 63.98±191.36 | 0.884 | 68.07±289.80 | 0.039* | 0.199 |
| Post | 60.77±118.03 | 0.884 | 41.32±85.63 | |||
| Total bilirubin (mg/dL) | Pre | 1.46±4.15 | 0.191 | 0.89±1.79 | 0.385 | 0.003** |
| Post | 1.82±4.82 | 0.191 | 0.96±2.23 | |||
| Albumin (g/dL) | Pre | 3.12±0.84 | 0.001** | 3.42±0.78 | 0.000*** | 0.000*** |
| Post | 2.78±0.61 | 0.001** | 2.98±0.54 | |||
| Total protein (g/dL) | Pre | 5.77±1.00 | 0.658 | 6.04±1.00 | 0.000*** | 0.007** |
| Post | 5.82±1.11 | 0.658 | 5.89±0.82 | |||
| C-reactive protein (mg/dL) | Pre | 11.37±10.78 | 0.005** | 9.56±9.79 | 0.000*** | 0.000*** |
| Post | 7.75±6.70 | 0.005** | 7.56±7.67 | |||
| Glucose (mg/dL) | Pre | 151.43±62.86 | 0.557 | 158.75±95.75 | 0.000*** | 0.000*** |
| Post | 142.63±137.54 | 0.557 | 124.50±68.42 | |||
| Triglyceride (mg/dL) | Pre | 116.40±76.95 | 0.019* | 118.42±99.50 | 0.004** | 0.019* |
| Post | 147.20±114.35 | 0.019* | 136.76±139.16 | |||
| Clinical effect | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| ICU LOS (d) | 21.16±15.83 | 16.48±11.06 | 0.007** |
| Hospital LOS (d) | 35.94±30.75 | 27.34±19.09 | 0.0107* |
| 30-Days mortality | 19 (20.0) | 56 (10.0) | 0.0347* |
| Hospital mortality | 25 (26.3) | 73 (13.0) | 0.0017** |
고 찰
결 론
- 1. Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, et al. Molecular mechanisms of glutamine action. J Cell Physiol 2005;204(2):392-401. ArticlePubMed
- 2. Curi R, Newsholme P, Marzuca-Nassr GN, Takahashi HK, Hirabara SM, Cruzat V, et al. Regulatory principles in metabolism-then and now. Biochem J 2016;473(13):1845-57. ArticlePubMedPDF
- 3. Cruzat VF, Pantaleão LC, Donato J Jr, de Bittencourt PI Jr, Tirapegui J. Oral supplementations with free and dipeptide forms of L-glutamine in endotoxemic mice:effects on muscle glutamine-glutathione axis and heat shock proteins. J Nutr Biochem 2014;25(3):345-52. ArticlePubMed
- 4. Roth E. Nonnutritive effects of glutamine. J Nutr 2008;138(10):2025S-31S. ArticlePubMed
- 5. Labow BI, Souba WW, Abcouwer SF. Mechanisms governing the expression of the enzymes of glutamine metabolism--glutaminase and glutamine synthetase. J Nutr 2001;131(9 Suppl):2467S-74S; discussion 2486S-7S. ArticlePubMed
- 6. Cruzat V, Newsholme P. An introduction to glutamine metabolism. In: Meynial-Denis D, editor. Glutamine:biochemistry, physiology, and clinical applications. Boca Raton: CRC Press; 2017. p. 1-18. Article
- 7. Park HJ. Role of parenteral glutamine in nutrition support for critically ill patients. J Clin Nutr 2015;7(2):42-8. Article
- 8. McClave SA. In: A.S.P.E.N. Board of Directors, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al, editors. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient:Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33(3):277-316. PubMed
- 9. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN guidelines on parenteral nutrition:intensive care. Clin Nutr 2009;28(4):387-400. ArticlePubMed
- 10. Critical Care Nutrition. 9.4 composition of parenteral nutrition:glutamine supplementation [Internet]. Ontario: Critical Care Nutrition; 2009. [[cited 2018 Apr 23]]. Available from: https:// www.criticalcarenutrition.com/docs/cpg/9.4pnglu_FINAL.pdf
- 11. Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997;13(4):295-302. ArticlePubMed
- 12. Goeters C, Wenn A, Mertes N, Wempe C, Van Aken H, Stehle P, et al. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit Care Med 2002;30(9):2032-7. ArticlePubMed
- 13. Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients:the French controlled, randomized, double-blind, multicenter study. Crit Care Med 2006;34(3):598-604. PubMed
- 14. Wernerman J, Kirketeig T, Andersson B, Berthelson H, Ersson A, Friberg H, et al. Scandinavian Critical Care Trials Group. Scandinavian glutamine trial:a pragmatic multi-centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiol Scand 2011;55(7):812-8. ArticlePubMed
- 15. Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Scottish Intensive care Glutamine or seleNium Evaluative Trial Trials Group. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542.ArticlePubMed
- 16. Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368(16):1489-97. ArticlePubMed
- 17. van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU:a randomized clinical trial. JAMA 2014;312(5):514-24. ArticlePubMed
- 18. Critical Care Nutrition. 9.4a composition of parenteral nutrition:glutamine supplementation [Internet]. Ontario: Critical Care Nutrition; 2015. [[cited 2018 Apr 23]]. Available from: http://www.criticalcarenutrition.com/docs/CPGs%202015/9.4a%202015.pdf
- 19. Lee HS, Kim S, Min YS, Sohn UD. Change of clinical effect upon use of glutamine to critically ill patients over age 60 receiving TPN. Korean J Clin Pharm 2014;24(1):9-14.
- 20. Critiacl Care Nutrition. 9.4a Composition of parenteral nutrition:glutamine supplementation [Internet]. Ontario: Critiacal Care Nutrition; 2013. [[cited 2018 Apr 23]]. Available from: https://www.criticalcarenutrition.com/docs/cpgs2012/9.4a.pdf
References
Figure & Data
REFERENCES
Citations

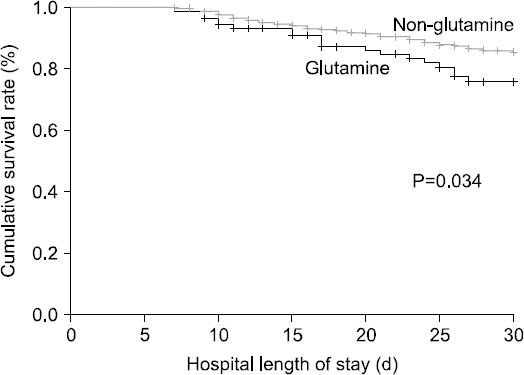
Fig. 1
Clinical characteristics of study group
| Characteristic | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| Gender | 0.296 | ||
| Male | 63 (66.3) | 341 (60.7) | |
| Female | 32 (33.7) | 221 (39.3) | |
| Age (y) | 66.11±15.78 | 67.75±15.47 | 0.341 |
| Height (cm) | 166.57±8.62 | 163.61±10.08 | 0.008 |
| Weight (kg) | 61.35±12.15 | 61.38±12.77 | 0.984 |
| Admission type | 0.000 |
||
| NS, NR | 20 (21.1) | 256 (45.6) | |
| GS, GE | 18 (18.9) | 44 (7.8) | |
| HE | 1 (1.1) | 3 (0.5) | |
| RP | 42 (44.2) | 113 (20.1) | |
| NH, UR | 1 (1.1) | 43 (7.7) | |
| Etc. | 13 (13.7) | 103 (18.3) | |
| APACHE II score at ICU entry | 6.00±7.36 | 6.26±6.58 | 0.720 |
Values are presented as number (%) or mean±standard deviation.
NS = neurosurgery; NR = neurology; GS = general surgery; GE = gastroenterology; HE = hematology; RP = respiratory medicine; NH = nephrology; UR = urology; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit.
**P<0.01,
***P<0.001.
Glutamine administration days and dose
| Glutamine administration (L-alanyl-L-glutamine) | Glutamine for 3 days or more (n=95) |
|---|---|
| Day | 10.12±8.93 |
| Dose (g/kg/d) |
0.33±0.10 |
Values are presented as mean±standard deviation.
aGuideline statement of intravenous glutamine supplementation dose: 0.3∼0.6 g/kg/day L-alanyl-L-glutamine.9
Comparison of laboratory results between two group at baseline
| Laboratory result | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| Serum creatinine (mg/dL) | 2.48±11.25 | 2.08±7.48 | 0.660 |
| Blood urea nitrogen (mg/dL) | 29.06±33.07 | 26.26±25.48 | 0.346 |
| Aspartate aminotransferase (U/L) | 90.15±295.13 | 123.44±574.60 | 0.581 |
| Alanine transaminase (U/L) | 63.50±190.39 | 67.29±287.76 | 0.902 |
| Total bilirubin (mg/dL) | 1.46±4.15 | 0.89±1.78 | 0.186 |
| Albumin (g/dL) | 3.12±0.84 | 3.41±0.78 | 0.001 |
| Total protein (g/dL) | 5.77±1.00 | 6.04±1.00 | 0.020 |
| C-reactive protein (mg/dL) | 11.48±10.77 | 9.56±9.76 | 0.085 |
| Glucose (mg/dL) | 151.43±62.86 | 158.83±95.53 | 0.334 |
| Triglyceride (mg/dL) | 116.4±76.95 | 118.83±99.33 | 0.825 |
Values are presented as mean±standard deviation.
*P<0.05,
**P<0.01.
Comparison of laboratory results between two group
| Laboratory result | Glutamine for 3 days or more (n=95) | P-value | Non-glutamine (n=562) | P-value | P-value | |
|---|---|---|---|---|---|---|
| Serum creatinine (mg/dL) | Pre | 2.47±11.25 | 0.329 | 2.09±7.52 | 0.012 |
0.062 |
| Post | 1.33±1.44 | 0.329 | 1.26±1.70 | |||
| Blood urea nitrogen (mg/dL) | Pre | 29.06±33.07 | 0.128 | 26.24±25.64 | 0.048 |
0.022 |
| Post | 35.27±28.21 | 0.128 | 29.24±28.29 | |||
| Aspartate aminotransferase (U/L) | Pre | 90.15±295.13 | 0.972 | 124.64±578.72 | 0.065 | 0.283 |
| Post | 88.92±188.73 | 0.972 | 73.42±294.86 | |||
| Alanine transaminase (U/L) | Pre | 63.98±191.36 | 0.884 | 68.07±289.80 | 0.039 |
0.199 |
| Post | 60.77±118.03 | 0.884 | 41.32±85.63 | |||
| Total bilirubin (mg/dL) | Pre | 1.46±4.15 | 0.191 | 0.89±1.79 | 0.385 | 0.003 |
| Post | 1.82±4.82 | 0.191 | 0.96±2.23 | |||
| Albumin (g/dL) | Pre | 3.12±0.84 | 0.001 |
3.42±0.78 | 0.000 |
0.000 |
| Post | 2.78±0.61 | 0.001** | 2.98±0.54 | |||
| Total protein (g/dL) | Pre | 5.77±1.00 | 0.658 | 6.04±1.00 | 0.000 |
0.007 |
| Post | 5.82±1.11 | 0.658 | 5.89±0.82 | |||
| C-reactive protein (mg/dL) | Pre | 11.37±10.78 | 0.005 |
9.56±9.79 | 0.000 |
0.000 |
| Post | 7.75±6.70 | 0.005** | 7.56±7.67 | |||
| Glucose (mg/dL) | Pre | 151.43±62.86 | 0.557 | 158.75±95.75 | 0.000 |
0.000 |
| Post | 142.63±137.54 | 0.557 | 124.50±68.42 | |||
| Triglyceride (mg/dL) | Pre | 116.40±76.95 | 0.019 |
118.42±99.50 | 0.004 |
0.019 |
| Post | 147.20±114.35 | 0.019* | 136.76±139.16 | |||
Values are presented as mean±standard deviation.
*P<0.05,
**P<0.01,
***P<0.001.
Comparison of hospital LOS, ICU LOS, and mortality
| Clinical effect | Glutamine for 3 days or more (n=95) | Non-glutamine (n=562) | P-value |
|---|---|---|---|
| ICU LOS (d) | 21.16±15.83 | 16.48±11.06 | 0.007 |
| Hospital LOS (d) | 35.94±30.75 | 27.34±19.09 | 0.0107 |
| 30-Days mortality | 19 (20.0) | 56 (10.0) | 0.0347 |
| Hospital mortality | 25 (26.3) | 73 (13.0) | 0.0017 |
Values are presented as mean±standard deviation or number (%).
LOS = length of stay; ICU = intensive care unit.
*P<0.05,
**P<0.01.
Values are presented as number (%) or mean±standard deviation. NS = neurosurgery; NR = neurology; GS = general surgery; GE = gastroenterology; HE = hematology; RP = respiratory medicine; NH = nephrology; UR = urology; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit. P<0.01, P<0.001.
Values are presented as mean±standard deviation. Guideline statement of intravenous glutamine supplementation dose: 0.3∼0.6 g/kg/day L-alanyl-L-glutamine.
Values are presented as mean±standard deviation. P<0.05, P<0.01.
Values are presented as mean±standard deviation. P<0.05, P<0.01, P<0.001.
Values are presented as mean±standard deviation or number (%). LOS = length of stay; ICU = intensive care unit. P<0.05, P<0.01.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite

